Figure 4.
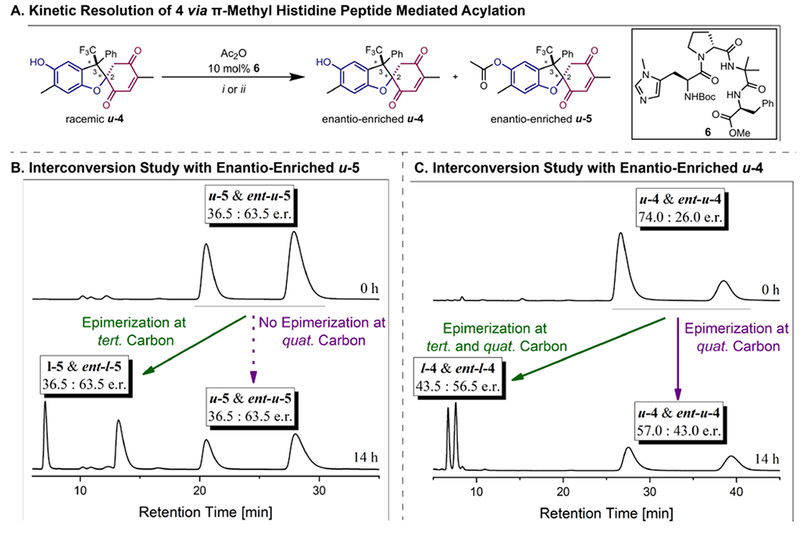
Studies of the stereodynamic dihydrobenzofuran network. A. Acylation of diastereopure u-4 with peptide catalyst 6. i) 0.6 equiv. Ac2O, CH2Cl2:toluene (1:5, v/v), −45 °C, 21 h, 19%. ii) 1.1 equiv. Ac2O, CHCl3:toluene (1:2, v/v), −18 °C, 40 h, 91%. B: Interconversion study of enantio-enriched u-5 in Hünig’s base/toluene (1:10, v/v) at RT. CHIRALPAK® IC (250 mm, i.d. 4.6 mm, particle size 5 μm), hexanes/iPrOH (90:10, v/v), 1.5 mL min−1, I-5 enantiomers: = 7.09 min and = 13.57 min, u-5 enantiomers: = 20.41 min and = 28.01 min. HPLC traces after 14 h and 34 h (see SI). C: Interconversion study of enantio-enriched u-4 in Hünig’s base/toluene (1:10, v/v) at RT. CHIRALPAK® IC (250 mm, i.d. 4.6 mm, particle size 5 μm), hexanes/iPrOH (95:5, v/v), 1.5 mL min−1, I-4 enantiomers: = 6.75 min and = 7.60 min, u-4 enantiomers: = 27.79 min and = 39.73 min. HPLC traces after 14 h and 34 h (see SI).
