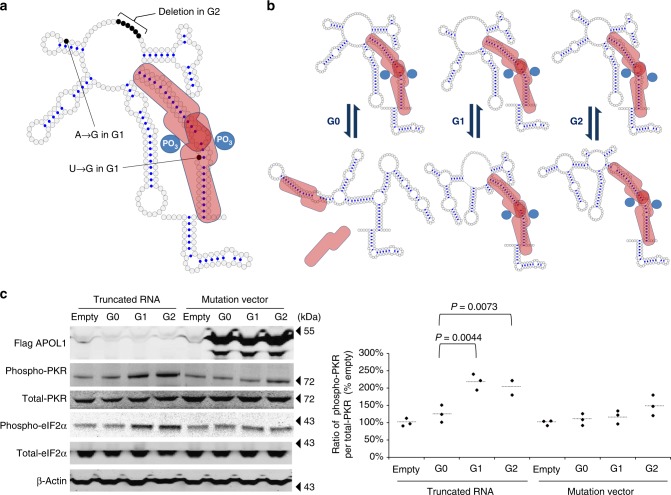
Fig. 3.
APOL1 RNA secondary structure serving as a scaffold for tandem PKR binding. a We generated lowest-energy secondary structural models for the truncated APOL1 G0 RNA variant (NM_001136540.1, 1180–1453) using RNAstructure software with SHAPE-derived reactivity profiles. Sequence differences between G0 and the G1 and G2 RNA variants are indicated here for convenience, although it should be noted that G1 and G2 variants do not occur on the same chromosome. A bipartite PKR is depicted in red to reflect the presence of distinct RNA binding and kinase domains in each protein monomer. Two PKR molecules are known to bind in tandem to long RNA duplexes, and this binding promotes autophosphorylation of PKR kinase domains and increases kinase activity. Blue dots mark sites at which Watson–Crick (G–C or A–T) or non-canonical (G–U) base pairing are predicted. We propose that the ~33 bp interrupted duplex motif within this segment of APOL1 RNA may serve as a docking site for tandem PKR binding. b Structure-based equilibrium model for APOL1 RNA-mediated PKR activation. SHAPE-derived secondary structural models of lowest energy (top) and second-lowest energy (bottom), along with their proposed interactions with PKR, are depicted. SHAPE and non-denaturing polyacrylamide gel electrophoresis indicate that APOL1 RNAs can assume alternative low energy conformations that may exist in a dynamic equilibrium. For APOL1 G0, the second-lowest energy model structure lacks a second PKR docking site, effectively reducing the number of such sites available for PKR binding autophosphorylation in a heterogeneous mixture of G0 RNA conformers and thus reducing PKR activation. This is in contrast to the proposed equilibrium states of the G1 and G2 RNAs, wherein both low energy conformers contain PKR docking sites and would therefore be expected to support PKR activation to a greater extent. c We generated stably-transfected HEK293FT cell lines expressing truncated APOL1 RNA which contain the APOL1 G0, G1, or G2 allele together with mutated variants whose secondary structure is disrupted by eight synonymous mutations. The mutated RNAs failed to promote PKR phosphorylation. All results are presented as ratio of empty, normalized to 100%. P values were calculated using a Student one-tailed t-test. Each horizontal line represents mean