Abstract
Histone deacetylase inhibitors (HDACi) can reverse chemoresistance, enhance chemotherapy-induced cytotoxicity, and reduce sarcoma proliferation in cell lines and animal models. We sought to determine the safety and toxicity of mocetinostat and its ability to reverse chemoresistance when administered with gemcitabine in patients with metastatic leiomyosarcoma resistant to prior gemcitabine-containing therapy. Participants with metastatic leiomyosarcoma received mocetinostat orally, 70 mg per day, three days per week, increasing to 90 mg after three weeks if well tolerated. Gemcitabine was administered at 1,000 mg/m2 intravenously at 10 mg/m2/minute on days five and 12 of every 21-day cycle. Disease response was evaluated with CT or MRI. Twenty participants with leiomyosarcoma were evaluated for toxicity. Median time to disease progression was 2.0 months (95% CI 1.54–3.12). Eighteen participants were evaluated for radiologic response by RECIST 1.1. Best responses included one PR and 12 SD. Tumor size reduced in 3 patients. Most common toxicities were fatigue, thrombocytopenia, anemia, nausea, and anorexia. One patient experienced a significant pericardial adverse event. No study-related deaths were observed. Rechallenging with gemcitabine by adding mocetinostat was feasible and demonstrated modest activity in patients with leiomyosarcoma. Further studies are needed to better define the role of HDAC inhibitors in patients with metastatic leiomyosarcoma.
1. Introduction
Leiomyosarcoma is a relatively common histologic subtype of soft tissue sarcoma that is usually incurable after development of metastasis [1]. Although cytotoxic chemotherapies such as doxorubicin [2–4], gemcitabine [5–7], and docetaxel [5, 8] can provide temporary benefit in some patients with metastatic leiomyosarcoma, these agents have modest clinical effectiveness [9, 10]. While gemcitabine does have single agent activity in leiomyosarcoma, combining docetaxel with gemcitabine yielded improvements in response rates, as well as progression-free and overall survival [11–15]. The most recent agents approved by the FDA for treatment of this disease include the multityrosine kinase inhibitor, pazopanib (Votrient), and a DNA binder, trabectedin (Yondelis) [16–18]. Neither of these drugs was shown to improve overall survival [19, 20]. Therefore, more effective treatments are needed.
We were interested in the role histone acetylation/deacetylation plays as a potential treatment strategy for sarcomas that are typically insensitive to traditional chemotherapeutic agents. Transcriptionally active genes are associated with hyperacetylated chromatin, while transcriptionally silent genes are associated with hypoacetylated chromatin [21, 22]. Chromatin acetylation is controlled by the opposite effects of two families of enzymes: histone acetyltransferases (HATs) and histone deacetylases (HDACs). HATs, as transcription coactivators, catalyze the addition of acetyl groups on the amino group of lysine residues in the N-terminal tails of core histones. Conversely, HDACs, as transcription corepressors, remove the acetyl groups from the acetylated lysines in histones [23]. Deregulation of HDAC activity can cause malignant diseases in humans [24].
Small molecule inhibitors of HDACs have emerged as a therapeutic class of molecules with anticancer potential [25, 26]. Anticancer activity of HDACi is mediated by regulating aberrant gene expression at the transcriptional level in cancer cells. These gene expression changes lead to inhibition of proliferation, induction of apoptosis, and/or cell differentiation in cancer cells in vitro and in vivo. Inhibitors of histone deacetylase (HDACi) have demonstrated preclinical activity in sarcoma models [27–36], and there have been anecdotes of patients with sarcoma responding to HDACi therapy [37]. There has been recent interest in using HDACi as synergistic therapy with chemotherapy for sarcomas [28,38–46]. We have recently completed a phase I study of an HDACi with chemotherapy in patients with metastatic sarcoma [47].
Histone deacetylase inhibitors have been observed to enhance apoptosis of cancer cells induced by several chemotherapeutic agents including gemcitabine [43,48–53]. Mocetinostat (MGCD0103) is an orally bio-available drug that has significant antitumor activity in vivo against a broad spectrum of human cancer types, and antitumor activity is achieved at clinically achievable doses [54–56]. Mocetinostat interacts synergistically with gemcitabine to inhibit cancer cell growth in vitro and in vivo [54, 56, 57]. These results suggest that a combination regimen with the HDAC inhibitor mocetinostat and gemcitabine may be a valuable therapeutic strategy to reverse chemoresistance in patients with gemcitabine-resistant leiomyosarcoma.
2. Materials and Methods
The study was an open label multicenter Phase II trial conducted as a part of the SARC (Sarcoma Alliance for Research through Collaboration) SPORE grant (U54CA168512) and registered on clinicaltrials.gov under the NLM identifier NCT02303262. The study protocol and consent forms were reviewed and approved by each of the participating institutions' institutional review boards. All patients participated in informed consent procedures prior to screening for eligibility. The patient group was comprised of adult patients who were diagnosed with leiomyosarcoma. As all patients were enrolled at academic centers of sarcoma excellence participating in the SPORE project, no central re-review of pathology was required. These patients had previously demonstrated disease progression by RECIST 1.1 either while receiving gemcitabine or within six months after completing a course of chemotherapy using a gemcitabine-based regimen. No limits on numbers of prior therapy were required for study entry.
After participants were confirmed eligible to participate in the study, each received 70 mg mocetinostat (provided by Mirati Therapeutics, Inc.) per day for three days per week in combination with gemcitabine, administered at 1000 mg/m2 at a rate of 10 mg/m2/minute [58–60] on days five and 12 of each 21-day cycle. The dose for mocetinostat was escalated to 90 mg/dose starting with cycle two if no grade three or four clinically significant toxicities or any new pericardial effusions were observed during the first cycle.
Because pericardial adverse events have been reported with mocetinostat treatment, participants underwent ECG screening on days one, five, and 12 of the first cycle, and cardiac ultrasound at screening, day 12 of cycles one and two, and before each subsequent cycle of therapy.
Study participants underwent CT imaging of the chest, abdomen, and pelvis prior to beginning cycle one and then every two cycles until disease progression; at which point, they were removed from study treatment.
All patients (including those who discontinued early) were followed for adverse events from enrollment into the study to at least 30 days after removal from the study or until death. The participants who were removed from study for unacceptable adverse events were followed until resolution or stabilization of the adverse event.
2.1. Statistical Design
This study was designed as a two-stage phase II clinical trial. Analysis was planned to be performed after 20 patients were enrolled (completion of stage I) to determine whether an additional 20 patients (stage II) should be enrolled. The primary objective of the study is to determine the rate of tumor response to treatment with mocetinostat and gemcitabine. Response to therapy was assessed by CT or MRI using RECIST 1.1 criteria. Response rates (CR or PR) were calculated as the number of patients achieving a response divided by the number of patients having been evaluated for response. 95% confidence intervals (CI) based on the binomial distribution were calculated. The Kaplan–Meier estimator was used to summarize the progression-free survival (PFS).
We would have considered combination therapy with mocetinostat and gemcitabine to be worthy of further evaluation if the true response rate was >20%, with the null hypothesis being that a <5% response rate would be seen with an ineffective treatment. Assuming that the number of successes is binomially distributed, this study had a one-sided alpha of 0.05 and a power of 0.92 for detecting a true clinical benefit rate of at least 20% versus the null hypothesis of 5% or less. This translated to a decision rule that the second stage of patients would be accrued if one or more responses (CR or PR) were seen in the first 20 patients. The treatment regimen would have been declared worthy of further study if five or more out of 40 patients had a response.
All patients who had initiated treatment were considered evaluable for adverse event analysis. The maximum grade of each adverse event was recorded for each patient and frequency tables for each adverse event observed were generated.
3. Results
A total of 20 patients (all with prior tumor growth after gemcitabine-containing therapy) were enrolled across five participating sites during the first stage. There were 18 patients evaluable for radiologic response. Eight of these patients had uterine leiomyosarcoma. Two patients withdrew consent prior to being evaluated for response. Reasons for withdrawal were to initiate hospice care and to avoid further adverse events the patients were experiencing.
Table 1 displays patient demographics and other characteristics at baseline. The median age was 57.5 years old (range: 39–71 years old). All patients had metastatic sarcoma at the time of enrollment in the trial and had been previously treated with a gemcitabine-containing regimen.
Table 1.
Patient characteristics (total number of patients = 20).
| Variable | N (%) | |
|---|---|---|
| Site | ||
| Dana-Farber Cancer Institute | 3 (15) | |
| Massachusetts General Hospital | 3 (15) | |
| Memorial Sloan Kettering Cancer Center | 5 (25) | |
| Ohio State University | 6 (30) | |
| University of Michigan | 3 (15) | |
|
| ||
| Sex | ||
| Female | 14 (70) | |
| Male | 6 (30) | |
|
| ||
| Ethnicity | ||
| Hispanic or Latino | 1 (5) | |
| Not Hispanic or Latino | 19 (95) | |
|
| ||
| Race | ||
| Asian | 2 (10) | |
| Black or African heritage | 3 (15) | |
| White | 15 (75) | |
|
| ||
| Tumor location at diagnosis | ||
| Kidney | 2 (10) | |
| Liver | 1 (5) | |
| Others | 6 (30) | |
| Pelvis | 1 (5) | |
| Peritoneum | 2 (10) | |
| Uterus | 8 (40) | |
|
| ||
| Metastasis present at diagnosis | ||
| No | 16 (80) | |
| Yes | 4 (20) | |
|
| ||
| Site of metastasis | ||
| Abdomen | 1 (5) | |
| Colon | 2 (10) | |
| Kidney | 1 (5) | |
| Liver | 4 (20) | |
|
| ||
| Lung | 12 (60) | |
| Pancreas | 1 (5) | |
| Pelvis | 2 (10) | |
| Peritoneum | 1 (5) | |
| Spine | 3 (15) | |
| Thyroid | 1 (5) | |
| Others | 3 (15) | |
| More than one site of metastasis | 6 (30) | |
|
| ||
| Received prior radiation therapy | ||
| No | 14 (70) | |
| Yes | 6 (30) | |
|
| ||
| Received prior surgery | ||
| No | 3 (15) | |
| Yes | 17 (85) | |
|
| ||
| Prior regimens | ||
| Gemcitabine/docetaxel | 20 (100) | |
| Gemcitabine/vinorelbine | 1 (5) | |
| AIM (doxorubicin, ifosfamide, and mesna) | 3 (15) | |
| Dacarbazine | 5 (25) | |
| Doxorubicin | 9 (45) | |
| Ifosfamide | 1 (5) | |
| Pazopanib | 7 (35) | |
| Trabectedin | 1 (5) | |
| Others | 10 (50) | |
| Number of prior lines of therapy before study entry | ||
| One | 6 | |
| Two | 3 | |
| Three | 2 | |
| Four | 4 | |
| Five | 2 | |
| Six | 1 | |
| Eight | 2 | |
All patients received at least one dose of mocetinostat and were evaluable for adverse events. A summary of adverse events (AEs), regardless of treatment attribution, is given in Table 2. Overall, 18 (90%) patients experienced a grade three or four AE: 11 (55%) patients experienced a nonhematological and 14 (70%) experienced a hematological grade three or four AEs. The most common AEs were fatigue, thrombocytopenia, anemia, and neutropenia. No study-related deaths were observed. Six patients had trace to minimal pericardial effusions. One patient had a small pericardial effusion, which was grade two. One patient experienced grade three pericardial adverse events. This was a 46-year-old woman who was hospitalized after grade three pericardial effusion which was seen on cardiac ultrasound on cycle one, day 12. This resulted in pericarditis, early cardiac tamponade, and pleural effusions. A summary of significant cardiac ultrasound findings is given in Table 3.
Table 2.
All adverse events regardless of attribution (N = 20 patients).
| Adverse events, n (%) | Grade 1 | Grade 2 | Grade 3 | Grade 4 |
|---|---|---|---|---|
| Anemia | 1 (5) | 4 (20) | 5 (25) | 1 (5) |
| Fatigue | 5 (25) | 5 (25) | 3 (15) | |
| Neutropenia | 6 (30) | 3 (15) | ||
| Thrombocytopenia | 3 (15) | 2 (10) | ||
| Peripheral sensory neuropathy | 1 (5) | 2 (10) | ||
| Lymphopenia | 1 (5) | 2 (10) | ||
| Diarrhea | 3 (15) | 1 (5) | ||
| Decreased ejection fraction | 1 (5) | |||
| Hypokalemia | 1 (5) | |||
| Pneumonia | 1 (5) | |||
| Noncardiac chest pain | 1 (5) | |||
| Pain | 1 (5) | |||
| Pericardial effusion∗ | 1 (5) | |||
| Pericardial tamponade∗ | 1 (5) | |||
| Pericarditis∗ | 1 (5) | |||
| Pulmonary embolism | 1 (5) | |||
| Syncope | 1 (5) | |||
| Vomiting | 4 (20) | 1 (5) | ||
| Leukopenia | 1 (5) | 2 (10) | ||
| Biopsy-related bleeding | 1 (5) |
∗Grade 3 pericardial effusion, tamponade, and pericarditis all occurred in the same patient.
Table 3.
Significant cardiac ultrasound findings.
| Patient | Ejection fraction at baseline (%) | Ejection fraction range (%) | Notable changes |
|---|---|---|---|
| 1 | 61 | 57–63 | |
| 2 | 59 | 58–64 | C1D1: there was mild eccentric left ventricular hypertrophy. There is mild diastolic dysfunction (impaired relaxation pattern with normal filling pressure) C2D1: there was mild diastolic dysfunction (impaired relaxation pattern with normal filling pressure) |
| 3 | 66 | 55–66 | |
| 4 | 65 | 55–65 | |
| 5 | 65 | 60–65 | |
| 6 | 64 | 60–64 | C1D12: grade 3 pericardial effusion, pericarditis, and tamponade |
| 7 | 76 | 76–81 | |
| 8 | 76 | 67–76 | C1D12: mild eccentric left ventricular hypertrophy (increased left ventricular mass with normal relative wall thickness) C2D1: (1) small left ventricular cavity size suggestive of an underfilled left ventricle; (2) there is mild diastolic dysfunction (impaired relaxation pattern with normal filling pressures) |
| 9 | 55 | 50–59 | |
| 10 | 69 | 69–73 | |
| 11 | 55 | 55–65 | |
| 12 | 68 | 68–74 | |
| 13 | 55 | 38–55 | C3D1: grade 3 reduction in cardiac ejection fraction |
| 14 | 67 | 66–69 | C4D1: grade 2 pericardial effusion |
| 15 | 58 | 55–60 | |
| 16 | 63 | 60–63 | |
| 17 | 59 | 59–60 | |
| 18 | 55 | 55–65 | |
| 19 | 64 | 61–69 | |
| 20 | 60 | 60–60 |
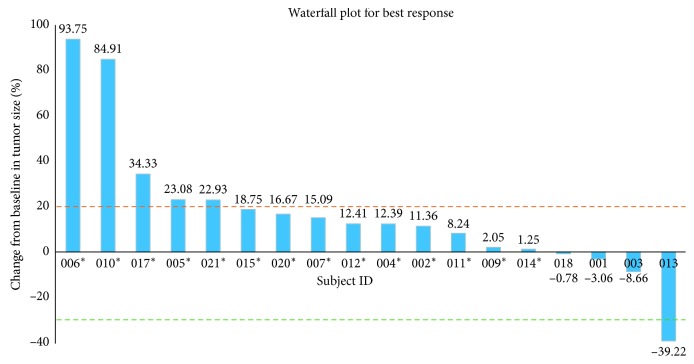
In the 18 participants evaluable for radiologic response, there was one PR (seen in a patient with uterine leiomyosarcoma), five PD (two with uterine leiomyosarcoma, two with leiomyosarcoma in the peritoneum, and one in the retroperitoneum), and 12 SD (five of these patients had uterine leiomyosarcoma, two had renal leiomyosarcoma, and five with others) as best response (Figure 1), with two participants completing five cycles or more. The median progression-free survival was two months (95% CI: 1.5 to 3.1) (Figure 2). In the one participant who did experience a PR, her target lesion was a single lung nodule. When a study-required postdrug biopsy was attempted, she experienced a clinically significant pulmonary bleed that required ICU support. Although she eventually recovered from this episode, another lung biopsy was not attempted due to safety concerns. Therefore, pathologic evaluation to confirm metastases and evaluate pharmacodynamic parameters was not possible. Given this uncertainty and the limited clinical benefit, if any, that was observed for this patient, SARC and Mirati Therapeutics, Inc. determined that the modest response rate observed at interim analysis did not justify further enrollment, and the study was halted after completion of the first stage.
Figure 1.
Waterfall plot for best response. The subject IDs with “∗” are those with progressive disease in the study. “PR” means partial response. The dotted lines indicate a 30% reduction and 20% increase from baseline, which are the cutoff points that determine partial response and progressive disease, respectively. The value for each vertical bar is added.
Figure 2.
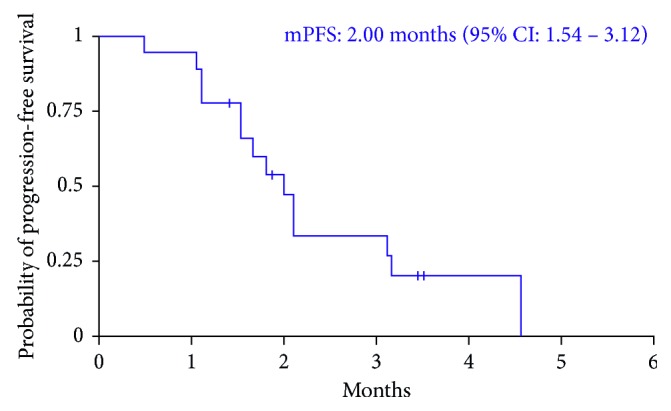
The Kaplan–Meier curve for progression-free survival.
4. Discussion
Numerous clinical trials have been performed to identify clinical activity of HDAC inhibitors in patients with solid tumors. Although HDAC inhibitors have shown success in preventing graft versus host disease (GVHD) [61] and treating patients with T-cell lymphoma [62] and multiple myeloma [63, 64]; to date, no study has shown significant benefit in patients with solid tumors [65, 66]. We sought to explore the potential for HDAC inhibition to reverse chemoresistance based on promising results seen in animal models of leiomyosarcoma. We chose mocetinostat as our model HDAC inhibitor because prior preclinical studies have shown synergy with gemcitabine [52] as measured by in vitro growth inhibition and apoptosis of PANC1 and BxPC3 pancreatic cancer cells. In a phase I/II study of mocetinostat and gemcitabine in patients with refractory solid tumors [67], the maximum tolerated dose of mocetinostat was 90 mg per dose, three doses per week, when administered with 1000 mg/m2 gemcitabine, given weekly for three weeks in every 28-day cycles. DLTs included fatigue, abdominal pain, deep vein thrombosis, diarrhea, nausea, mental status change, thrombocytopenia, and vomiting. The phase II portion of this study focused on patients with pancreatic cancer.
This dose and schedule of administration for our current study was modified from this previously conducted phase I/II study. The timing of administration of gemcitabine was modified to days 5 and 12 in order to ensure patients were predosed sufficiently with mocetinostat to allow for concurrent drug exposure to tumor cells. The previously published study administered gemcitabine on day 1 of each week. Given the short half-life of gemcitabine, this would not have allowed for prolonged coexposure of tumor cells to both agents simultaneously. Additionally, the prior study reported that 81% of all patients experienced grade 3 or greater treatment-related adverse events. Therefore, in designing the current study, we opted to start with a lower dose of mocetinostat at 70 mg for one cycle to evaluate tolerability before advancing to the MTD/RP2D dose of 90 mg reported in the prior study.
To show the relative benefits of adding mocetinostat to gemcitabine therapy would involve a large randomized clinical trial that would have been logistically challenging in sarcoma. Therefore, we initially focused our study on the selected patients who already demonstrated chemoresistance to gemcitabine. In such a cohort of patients, even a small number of tumor responses to therapy would be a significant proof of principle that mocetinostat could reverse chemoresistance as the null hypothesis is that no responses would be seen if mocetinostat was an inactive drug. This allowed us to test a relatively modest-size cohort for the proof of concept that mocetinostat would reverse gemcitabine chemoresistance.
We enrolled only patients with metastatic leiomyosarcoma who progressed either during treatment with gemcitabine or within six months of completing treatment with gemcitabine. The adverse events that we observed were largely expected and observed in prior clinical trials using mocetinostat. Pericardial SAEs, for example, were seen in 19 cases (4.3%) of the 435 patients who had previously received mocetinostat prior to this study. Based on this finding, we incorporated frequent cardiac ultrasound monitoring to be performed at screening, during cycle one, and at every cycle of the study. The current study observed six cases of trace to minimal and clinically insignificant pericardial effusion, one case of grade 2 pericardial effusion (listed in Table 3), and one case of significant grade 3 pericardial effusion that led to pericarditis and cardiac tamponade (listed in Table 3).
Overall, in the context that the subjects had metastatic disease that would be universally fatal unless an effective treatment can be identified, we found that the combination of mocetinostat and gemcitabine was relatively well tolerated. However, the median progression-free survival for participants was short, and we did not observe significant response rates as determined by RECIST.
5. Conclusions
Although we showed that mocetinostat can be safely combined with gemcitabine in this study population, our study did not demonstrate that mocetinostat can reverse chemoresistance in patients with previously established gemcitabine-resistant leiomyosarcoma. However, we studied only patients with metastatic leiomyosarcoma who had previously progressed on a gemcitabine-containing chemotherapy regimen. As these patients were selected for their chemoresistant tumor characteristics, our study observations do not negate a potential role for HDAC inhibitors as a synergistic mechanism in chemotherapy naïve patients or for patients with other solid tumors. As several minor responses were seen in this study, additional studies are needed to better define a role for HDAC inhibitors in patients with sarcoma.
Acknowledgments
This research was supported by funds and supply of mocetinostat from Mirati Therapeutics, Inc. The study was also supported by funding from the National Cancer Institute of the National Institutes of Health “SARC SPORE Grant” under Award Number U54CA168512.
Data Availability
The data used to support the findings of this study are available from the corresponding author upon request.
Disclosure
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Conflicts of Interest
Dr. Ballman reports grants from SARC Foundation during the conduct of the study. Dr. Choy reports grants from NCI during the conduct of the study and is a paid consultant for EMD Serrono, Daiichi, Amgen, and Bayer. Dr. S. Patel reports grants and personal fees from Janssen, grants and personal fees from Eisai, grants and personal fees from Morphotek, personal fees from EMD-Serono, personal fees from CytRx, personal fees from Bayer, personal fees from Eli Lilly, personal fees from Epizyme, and personal fees from Novartis, outside the submitted work. Dr. Chugh reports grants and others from Epizyme, Inc, others from EMD Serano, grants from AADi, grants from Novartis, grants from Lilly, grants from Medivation, Pfizer, grants from Morphotek, and grants from Mabvax, outside the submitted work.
References
- 1.Daigeler A., Zmarsly I., Hirsch T., et al. Long-term outcome after local recurrence of soft tissue sarcoma: a retrospective analysis of factors predictive of survival in 135 patients with locally recurrent soft tissue sarcoma. British Journal of Cancer. 2014;110(6):1456–1464. doi: 10.1038/bjc.2014.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khalek Y., Vilor M., Sorrentino J., Brown M., Wills J., Herrera L. Complete disappearance of a leiomyosarcoma of the lower extremity following preoperative hyperthermia and intra-arterial doxorubicin. Journal of Surgical Oncology. 1993;52(4):272–275. doi: 10.1002/jso.2930520418. [DOI] [PubMed] [Google Scholar]
- 3.Penel N., Italiano A., Isambert N., Bompas E., Bousquet G., Duffaud F. Factors affecting the outcome of patients with metastatic leiomyosarcoma treated with doxorubicin-containing chemotherapy. Annals of Oncology. 2010;21(6):1361–1365. doi: 10.1093/annonc/mdp485. [DOI] [PubMed] [Google Scholar]
- 4.Sutton G., Blessing J., Hanjani P., Kramer P. Phase II evaluation of liposomal doxorubicin (Doxil) in recurrent or advanced leiomyosarcoma of the uterus: a Gynecologic Oncology Group study. Gynecologic Oncology. 2005;96(3):749–752. doi: 10.1016/j.ygyno.2004.11.036. [DOI] [PubMed] [Google Scholar]
- 5.Gupta A. A., Yao X., Verma S., Mackay H., Hopkins L. Chemotherapy (gemcitabine, docetaxel plus gemcitabine, doxorubicin, or trabectedin) in inoperable, locally advanced, recurrent, or metastatic uterine leiomyosarcoma: a clinical practice guideline. Current Oncology. 2013;20(5):e448–e454. doi: 10.3747/co.20.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Look K. Y., Sandler A., Blessing J. A., Lucci J. A., 3rd, Rose P. G. phase II trial of gemcitabine as second-line chemotherapy of uterine leiomyosarcoma: a gynecologic oncology group (GOG) study. Gynecologic Oncology. 2004;92(2):644–647. doi: 10.1016/j.ygyno.2003.11.023. [DOI] [PubMed] [Google Scholar]
- 7.Silvestris N., Piscitelli D., Crucitta E., Fiore M., De Lena M., Lorusso V. Unusual response to second-line single-agent gemcitabine in locally advanced primary leiomyosarcoma of the lung: a case report. Journal of Chemotherapy. 2003;15(5):507–509. doi: 10.1179/joc.2003.15.5.507. [DOI] [PubMed] [Google Scholar]
- 8.Seddon B., Scurr M., Jones R. L., et al. A phase II trial to assess the activity of gemcitabine and docetaxel as first line chemotherapy treatment in patients with unresectable leiomyosarcoma. Clinical Sarcoma Research. 2015;5(1):p. 13. doi: 10.1186/s13569-015-0029-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gupta A. A., Yao X., Verma S., Mackay H., Hopkins L. Systematic chemotherapy for inoperable, locally advanced, recurrent, or metastatic uterine leiomyosarcoma: a systematic review. Clinical Oncology. 2013;25(6):346–355. doi: 10.1016/j.clon.2012.11.008. [DOI] [PubMed] [Google Scholar]
- 10.Kang S., Kim H. S., Kim S., Kim W., Han I. Post-metastasis survival in extremity soft tissue sarcoma: a recursive partitioning analysis of prognostic factors. European Journal of Cancer. 2014;50(9):1649–1656. doi: 10.1016/j.ejca.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 11.Dickson M. A., D’Adamo D. R., Keohan M. L., et al. Phase II trial of gemcitabine and docetaxel with bevacizumab in soft tissue sarcoma. Sarcoma. 2015;2015:7. doi: 10.1155/2015/532478.532478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hensley M. L., Maki R., Venkatraman E., et al. Gemcitabine and docetaxel in patients with unresectable leiomyosarcoma: results of a phase II trial. Journal of Clinical Oncology. 2002;20(12):2824–2831. doi: 10.1200/JCO.2002.11.050. [DOI] [PubMed] [Google Scholar]
- 13.Maki R. G. Gemcitabine and docetaxel in metastatic sarcoma: past, present, and future. Oncologist. 2007;12(8):999–1006. doi: 10.1634/theoncologist.12-8-999. [DOI] [PubMed] [Google Scholar]
- 14.Maki R. G., Wathen J. K., Patel S. R., et al. Randomized phase II study of gemcitabine and docetaxel compared with gemcitabine alone in patients with metastatic soft tissue sarcomas: results of sarcoma alliance for research through collaboration study 002 (corrected) Journal of Clinical Oncology. 2007;25(19):2755–2763. doi: 10.1200/JCO.2006.10.4117. [DOI] [PubMed] [Google Scholar]
- 15.Hensley M. L., Miller A., O’Malley D. M., et al. Randomized phase III trial of gemcitabine plus docetaxel plus bevacizumab or placebo as first-line treatment for metastatic uterine leiomyosarcoma: an NRG Oncology/Gynecologic Oncology Group study. Journal of Clinical Oncology. 2015;33(10):1180–1185. doi: 10.1200/JCO.2014.58.3781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ratan R., Patel S. R. Chemotherapy for soft tissue sarcoma. Cancer. 2016;122(19):2952–2960. doi: 10.1002/cncr.30191. [DOI] [PubMed] [Google Scholar]
- 17.Kawai A., Yonemori K., Takahashi S., Araki N., Ueda T. Systemic therapy for soft tissue sarcoma: proposals for the optimal use of pazopanib, trabectedin, and eribulin. Advances in Therapy. 2017;34(7):1556–1571. doi: 10.1007/s12325-017-0561-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martin Broto J., Le Cesne A., Reichardt P. The importance of treating by histological subtype in advanced soft tissue sarcoma. Future Oncology. 2017;13(1s):23–31. doi: 10.2217/fon-2016-0500. [DOI] [PubMed] [Google Scholar]
- 19.van der Graaf W. T., Blay J. Y., Chawla S. P., et al. Pazopanib for metastatic soft-tissue sarcoma (PALETTE): a randomised, double-blind, placebo-controlled phase 3 trial. The Lancet. 2012;379(9829):1879–1886. doi: 10.1016/S0140-6736(12)60651-5. [DOI] [PubMed] [Google Scholar]
- 20.Demetri G. D., von Mehren M., Jones R. L., et al. Efficacy and safety of trabectedin or dacarbazine for metastatic liposarcoma or leiomyosarcoma after failure of conventional chemotherapy: results of a phase III randomized multicenter clinical trial. Journal of Clinical Oncology. 2016;34(8):786–793. doi: 10.1200/JCO.2015.62.4734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Csordas A. On the biological role of histone acetylation. Biochemical Journal. 1990;265(1):23–38. doi: 10.1042/bj2650023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grunstein M. Histone acetylation in chromatin structure and transcription. Nature. 1997;389(6649):349–352. doi: 10.1038/38664. [DOI] [PubMed] [Google Scholar]
- 23.Hassig C. A., Schreiber S. L. Nuclear histone acetylases and deacetylases and transcriptional regulation: HATs off to HDACs. Current Opinion in Chemical Biology. 1997;1(3):300–308. doi: 10.1016/s1367-5931(97)80066-x. [DOI] [PubMed] [Google Scholar]
- 24.Kouzarides T. Histone acetylases and deacetylases in cell proliferation. Current Opinion in Genetics and Development. 1999;9(1):40–48. doi: 10.1016/s0959-437x(99)80006-9. [DOI] [PubMed] [Google Scholar]
- 25.Li Z., Zhu W. G. Targeting histone deacetylases for cancer therapy: from molecular mechanisms to clinical implications. International Journal of Biological Sciences. 2014;10(7):757–770. doi: 10.7150/ijbs.9067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lane A. A., Chabner B. A. Histone deacetylase inhibitors in cancer therapy. Journal of Clinical Oncology. 2009;27(32):5459–5468. doi: 10.1200/JCO.2009.22.1291. [DOI] [PubMed] [Google Scholar]
- 27.Hrzenjak A., Moinfar F., Kremser M. L., et al. Histone deacetylase inhibitor vorinostat suppresses the growth of uterine sarcomas in vitro and in vivo. Molecular Cancer. 2010;9(1):p. 49. doi: 10.1186/1476-4598-9-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sampson E. R., Amin V., Schwarz E. M., O’Keefe R. J., Rosier R. N. The histone deacetylase inhibitor vorinostat selectively sensitizes fibrosarcoma cells to chemotherapy. Journal of Orthopaedic Research. 2011;29(4):623–632. doi: 10.1002/jor.21274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yamamoto S., Tanaka K., Sakimura R., et al. Suberoylanilide hydroxamic acid (SAHA) induces apoptosis or autophagy-associated cell death in chondrosarcoma cell lines. Anticancer Research. 2008;28(3A):1585–1591. [PubMed] [Google Scholar]
- 30.Berghuis D., Schilham M. W., Vos H. I., et al. Histone deacetylase inhibitors enhance expression of NKG2D ligands in Ewing sarcoma and sensitize for natural killer cell-mediated cytolysis. Clinical Sarcoma Research. 2012;2(1):p. 8. doi: 10.1186/2045-3329-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Di Pompo G., Salerno M., Rotili D., et al. Novel histone deacetylase inhibitors induce growth arrest, apoptosis, and differentiation in sarcoma cancer stem cells. Journal of Medicinal Chemistry. 2015;58(9):4073–4079. doi: 10.1021/acs.jmedchem.5b00126. [DOI] [PubMed] [Google Scholar]
- 32.Ito T., Ouchida M., Morimoto Y., et al. Significant growth suppression of synovial sarcomas by the histone deacetylase inhibitor FK228 in vitro and in vivo. Cancer Letters. 2005;224(2):311–319. doi: 10.1016/j.canlet.2004.10.030. [DOI] [PubMed] [Google Scholar]
- 33.Liu S., Cheng H., Kwan W., Lubieniecka J. M., Nielsen T. O. Histone deacetylase inhibitors induce growth arrest, apoptosis, and differentiation in clear cell sarcoma models. Molecular Cancer Therapeutics. 2008;7(6):1751–1761. doi: 10.1158/1535-7163.MCT-07-0560. [DOI] [PubMed] [Google Scholar]
- 34.Lubieniecka J. M., de Bruijn D. R., Su L., et al. Histone deacetylase inhibitors reverse SS18-SSX-mediated polycomb silencing of the tumor suppressor early growth response 1 in synovial sarcoma. Cancer Research. 2008;68(11):4303–4310. doi: 10.1158/0008-5472.CAN-08-0092. [DOI] [PubMed] [Google Scholar]
- 35.Sakimura R., Tanaka K., Yamamoto S., et al. The effects of histone deacetylase inhibitors on the induction of differentiation in chondrosarcoma cells. Clinical Cancer Research. 2007;13(1):275–282. doi: 10.1158/1078-0432.CCR-06-1696. [DOI] [PubMed] [Google Scholar]
- 36.Sonnemann J., Dreyer L., Hartwig M., et al. Histone deacetylase inhibitors induce cell death and enhance the apoptosis-inducing activity of TRAIL in Ewing’s sarcoma cells. Journal of Cancer Research and Clinical Oncology. 2007;133(11):847–858. doi: 10.1007/s00432-007-0227-8. [DOI] [PubMed] [Google Scholar]
- 37.Lee J., McGuire C. Clinical efficacy of vorinostat in a patient with leiomyosarcoma. Clinical Medical Insights: Oncol. 2012;6:101–105. doi: 10.4137/CMO.S7194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lopez G., Bill K. L., Bid H. K., et al. HDAC8, a potential therapeutic target for the treatment of malignant peripheral nerve sheath tumors (MPNST) PLoS One. 2015;10(7) doi: 10.1371/journal.pone.0133302.e0133302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lopez G., Liu J., Ren W., et al. Combining PCI-24781, a novel histone deacetylase inhibitor, with chemotherapy for the treatment of soft tissue sarcoma. Clinical Cancer Research. 2009;15(10):3472–3483. doi: 10.1158/1078-0432.CCR-08-2714. [DOI] [PubMed] [Google Scholar]
- 40.Lopez G., Song Y., Lam R., et al. HDAC inhibition for the treatment of epithelioid sarcoma: novel cross talk between epigenetic components. Molecular Cancer Research. 2016;14(1):35–43. doi: 10.1158/1541-7786.MCR-15-0295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang C., Choy E., Hornicek F. J., et al. Histone deacetylase inhibitor PCI-24781 enhances chemotherapy-induced apoptosis in multidrug-resistant sarcoma cell lines. Anticancer Research. 2011;31(4):1115–1123. [PMC free article] [PubMed] [Google Scholar]
- 42.Yang C., Choy E., Hornicek F. J., et al. Histone deacetylase inhibitor (HDACI) PCI-24781 potentiates cytotoxic effects of doxorubicin in bone sarcoma cells. Cancer Chemotherapy and Pharmacology. 2011;67(2):439–446. doi: 10.1007/s00280-010-1344-7. [DOI] [PubMed] [Google Scholar]
- 43.Hurtubise A., Bernstein M. L., Momparler R. L. Preclinical evaluation of the antineoplastic action of 5-aza-2’-deoxycytidine and different histone deacetylase inhibitors on human Ewing’s sarcoma cells. Cancer Cell International. 2008;8(1):p. 16. doi: 10.1186/1475-2867-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nguyen A., Su L., Campbell B., Poulin N. M., Nielsen T. O. Synergism of heat shock protein 90 and histone deacetylase inhibitors in synovial sarcoma. Sarcoma. 2009;2009:10. doi: 10.1155/2009/794901.794901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sampson V. B., Vetter N. S., Kamara D. F., Collier A. B., Gresh R. C., Kolb E. A. Vorinostat enhances cytotoxicity of SN-38 and temozolomide in ewing sarcoma cells and activates STAT3/AKT/MAPK pathways. PLoS One. 2015;10(11) doi: 10.1371/journal.pone.0142704.e0142704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wittenburg L. A., Bisson L., Rose B. J., Korch C., Thamm D. H. The histone deacetylase inhibitor valproic acid sensitizes human and canine osteosarcoma to doxorubicin. Cancer Chemotherapy and Pharmacology. 2011;67(1):83–92. doi: 10.1007/s00280-010-1287-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Choy E., Flamand Y., Balasubramanian S., et al. Phase 1 study of oral abexinostat, a histone deacetylase inhibitor, in combination with doxorubicin in patients with metastatic sarcoma. Cancer. 2015;121(8):1223–1230. doi: 10.1002/cncr.29175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Candelaria M., de la Cruz-Hernandez E., Taja-Chayeb L., et al. DNA methylation-independent reversion of gemcitabine resistance by hydralazine in cervical cancer cells. PLoS One. 2012;7(3) doi: 10.1371/journal.pone.0029181.e29181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Donadelli M., Costanzo C., Beghelli S., et al. Synergistic inhibition of pancreatic adenocarcinoma cell growth by trichostatin A and gemcitabine. Biochimica et Biophysica Acta. 2007;1773(7):1095–1106. doi: 10.1016/j.bbamcr.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 50.Iwahashi S., Shimada M., Utsunomiya T., et al. Histone deacetylase inhibitor enhances the anti-tumor effect of gemcitabine: a special reference to gene-expression microarray analysis. Oncology Reports. 2011;26(5):1057–1062. doi: 10.3892/or.2011.1407. [DOI] [PubMed] [Google Scholar]
- 51.Iwahashi S., Shimada M., Utsunomiya T., et al. Histone deacetylase inhibitor augments anti-tumor effect of gemcitabine and pegylated interferon-alpha on pancreatic cancer cells. International Journal of Clinical Oncology. 2011;16(6):671–678. doi: 10.1007/s10147-011-0246-y. [DOI] [PubMed] [Google Scholar]
- 52.Sung V., Richard N., Brady H., Maier A., Kelter G., Heise C. Histone deacetylase inhibitor MGCD0103 synergizes with gemcitabine in human pancreatic cells. Cancer Science. 2011;102(6):1201–1207. doi: 10.1111/j.1349-7006.2011.01921.x. [DOI] [PubMed] [Google Scholar]
- 53.Tavallai S., Hamed H. A., Grant S., Poklepovic A., Dent P. Pazopanib and HDAC inhibitors interact to kill sarcoma cells. Cancer Biology and Therapy. 2014;15(5):578–585. doi: 10.4161/cbt.28163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Le Tourneau C., Siu L. L. Promising antitumor activity with MGCD0103, a novel isotype-selective histone deacetylase inhibitor. Expert Opinion on Investigational Drugs. 2008;17(8):1247–1254. doi: 10.1517/13543784.17.8.1247. [DOI] [PubMed] [Google Scholar]
- 55.Siu L. L., Pili R., Duran I., et al. Phase I study of MGCD0103 given as a three-times-per-week oral dose in patients with advanced solid tumors. Journal of Clinical Oncology. 2008;26(12):1940–1947. doi: 10.1200/JCO.2007.14.5730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhou N., Moradei O., Raeppel S., et al. Discovery of N-(2-aminophenyl)-4-[(4-pyridin-3-ylpyrimidin-2-ylamino)methyl]benzamide (MGCD0103), an orally active histone deacetylase inhibitor. Journal of Medicinal Chemistry. 2008;51(14):4072–4075. doi: 10.1021/jm800251w. [DOI] [PubMed] [Google Scholar]
- 57.Fournel M., Bonfils C., Hou Y., et al. MGCD0103, a novel isotype-selective histone deacetylase inhibitor, has broad spectrum antitumor activity in vitro and in vivo. Molecular Cancer Therapeutics. 2008;7(4):759–768. doi: 10.1158/1535-7163.MCT-07-2026. [DOI] [PubMed] [Google Scholar]
- 58.Hensley M. L., Blessing J. A., Degeest K., Abulafia O., Rose P. G., Homesley H. D. Fixed-dose rate gemcitabine plus docetaxel as second-line therapy for metastatic uterine leiomyosarcoma: a Gynecologic Oncology Group phase II study. Gynecologic Oncology. 2008;109(3):323–328. doi: 10.1016/j.ygyno.2008.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hensley M. L., Blessing J. A., Mannel R., Rose P. G. Fixed-dose rate gemcitabine plus docetaxel as first-line therapy for metastatic uterine leiomyosarcoma: a Gynecologic Oncology Group phase II trial. Gynecologic Oncology. 2008;109(3):329–334. doi: 10.1016/j.ygyno.2008.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tempero M., Plunkett W., Ruiz Van Haperen V., et al. Randomized phase II comparison of dose-intense gemcitabine: thirty-minute infusion and fixed dose rate infusion in patients with pancreatic adenocarcinoma. Journal of Clinical Oncology. 2003;21(18):3402–3408. doi: 10.1200/JCO.2003.09.140. [DOI] [PubMed] [Google Scholar]
- 61.Choi S., Reddy P. HDAC inhibition and graft versus host disease. Molecular Medicine. 2011;17(5-6):404–416. doi: 10.2119/molmed.2011.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.DeSimone J. A., Sodha P., Ignatova D., Dummer R., Cozzio A., Guenova E. Recent advances in primary cutaneous T-cell lymphoma. Current Opinion in Oncology. 2015;27(2):128–133. doi: 10.1097/CCO.0000000000000161. [DOI] [PubMed] [Google Scholar]
- 63.Afifi S., Michael A., Azimi M., Rodriguez M., Lendvai N., Landgren O. Role of histone deacetylase inhibitors in relapsed refractory multiple myeloma: a focus on vorinostat and panobinostat. Pharmacotherapy. 2015;35(12):1173–1188. doi: 10.1002/phar.1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Harada T., Hideshima T., Anderson K. C. Histone deacetylase inhibitors in multiple myeloma: from bench to bedside. International Journal of Hematology. 2016;104(3):300–309. doi: 10.1007/s12185-016-2008-0. [DOI] [PubMed] [Google Scholar]
- 65.Qin H. T., Li H. Q., Liu F. Selective histone deacetylase small molecule inhibitors: recent progress and perspectives. Expert Opinion on Therapeutic Patents. 2017;27(5):621–636. doi: 10.1080/13543776.2017.1276565. [DOI] [PubMed] [Google Scholar]
- 66.Zagni C., Floresta G., Monciino G., Rescifina A. The search for potent, small-molecule HDACIs in cancer treatment: a decade after vorinostat. Medicinal Research Reviews. 2017;37(6):1373–1428. doi: 10.1002/med.21437. [DOI] [PubMed] [Google Scholar]
- 67.Chan E., Chiorean E. G., O’Dwyer P. J., et al. Phase I/II study of mocetinostat in combination with gemcitabine for patients with advanced pancreatic cancer and other advanced solid tumors. Cancer Chemotherapy and Pharmacology. 2017;81(2):355–364. doi: 10.1007/s00280-017-3494-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.