Abstract
The promise of personalized medicine to deliver “the right treatments at the right time to the right person” is the next frontier in healthcare. However, to implement personalized medicine in chronic diseases such as diabetes mellitus and diabetic kidney disease (DKD), a number of different aspects need to be taken into account. Better risk stratification and more precise options for treatment need to be developed and included in clinical practice guidelines. A patient's unique psychological, social and environmental situation also drive disease progression and outcomes. Appraising the cost effectiveness of precision medicines is necessary, not just as the cost of new therapies, but also the cost of diagnosis with novel methodologies and averted complications. As the prevalence of DKD grows worldwide to epidemic proportions, challenges such as global disparities in resources, access to healthcare and prevalence need to be addressed. This review considers these issues to achieve the short and longer‐term goals of implementing personalized medicine in clinical practice.
Keywords: challenges, diabetes, implementation, kidney disease, personalized medicine
1. INTRODUCTION
Diabetes mellitus, and especially type 2 diabetes, will be the seventh leading cause of mortality by 2030.1 Diabetic kidney disease (DKD), a complication of diabetes, is the most common cause of end‐stage renal disease (ESRD) both in the developed and the developing world and accounts for 20% to 40% of patients starting renal replacement therapy.2 Often diabetes and DKD are coupled with hypertension and cardiovascular disease. Conventional treatment paradigms target risk factors separately whereas comorbidities are not necessarily independent of each other.
Over the last decades, there has been clear progress in assessing risk factors and controlling diabetes. Subsequently, the prognosis of patients with diabetes has gradually improved.1 Nonetheless, mortality, when compared to the general population, is higher, and the risk is especially high in patients with impaired renal function. Many reasons for the morbidity and mortality in DKD have been identified, including suboptimal application of evidence‐based therapies (eg, due to lack of medication intensification by physicians or insufficient lifestyle changes or medication adherence by patients), and variability in response to medication (eg, inadequate efficacy of therapy even when optimally applied, or genetic differences, leading to differential treatment response). Additionally, a general lack of understanding of the true pathobiology of DKD results in the treatment of symptoms and diagnostic labels instead of a focus on causes and mechanisms. This inefficiency results in many patients not being properly treated or not receiving the maximum benefit possible for the multitude of treatment modalities available. In order to improve the situation, a shift towards care of the individual patient is needed, rather than for the particular manifestation of the disease.
Individualized medicine is a medical model that proposes the customization of healthcare—with medical decisions, practices and/or products being tailored to the individual patient. Ultimately, this medical model aims to improve patient care and achieve better outcomes, all while providing a more cost effective healthcare system. Chronic diseases like diabetes will cost the global economy $47 trillion over the next 20 years. DKD additionally places a huge economic burden on the healthcare system. The overall costs of care for people with DKD are extraordinarily high, due in large part to the strong relationship of DKD with cardiovascular disease and development of ESRD.3 The total annual cost to the National Health Service (NHS) in the United Kingdom was £685 million ($1.5 billion),4 and the overall Medicare expenditures for diabetes and chronic kidney disease (CKD) in the mostly older (≥65 years of age) US Medicare population were approximately $25 billion in 2011.3 In view of these enormous costs of DKD, identifying strategies for better cost‐benefit is in the best interest of patients and society.
In this article, we highlight different aspects that need to be taken into account in order to individualize medicine in chronic diseases including diabetes and DKD. Clinical practice guidelines need to include stratifying risk more accurately and offer more precise treatment options. The patient as an individual, with his or her unique social and environment situations are also important drivers of disease progression and outcomes. How to evaluate the cost effectiveness of precision medicines is also necessary, but challenges in doing so are present. Finally, the prevalence of DKD is growing worldwide, with the fastest growth occurring in low‐income countries (LIC) and low‐middle‐income countries (LMIC). In order to implement personalized medicine for DKD on a global scale, a number of challenges first need to be addressed.
2. NEED TO INCLUDE INDIVIDUALIZED MEDICINE IN CLINICAL PRACTICE GUIDELINES
For decades, DKD was considered a disease with a uniform clinical course and pathophysiology. Evidence‐based guidelines have been developed based on findings obtained from large interventional trials, and indeed these guidelines have been critical to improving the overall quality of care of patients with DKD. Certainly, implementation of the recommendations in clinical practice resulted in considerable progress and benefit for the patients. However, growing consensus suggests that renal disease in patients with diabetes is increasingly complex and heterogeneous, and many patients do not follow the lowering renal function/increasing albuminuria paradigm. Furthermore, recent studies clearly show that the treatment response and risk of side effects differ between individuals,5 and even within individuals over time.6 These findings should modify our approach to DKD in general, recognizing that the variability of the disease should be taken into account when choosing therapy.
Currently, evidence for these guidelines is based on the effect of drugs on clinical outcomes on populations included in clinical trials rather than based on individuals. For example, in the TREAT study, patients with DKD and anemia were randomly assigned to darbepoetin alfa to achieve a hemoglobin level of 13 g/dL or to rescue erythropoiesis stimulating agent (ESA) therapy in case hemoglobin levels dropped to less than 9.0 g/dL. Active therapy did not reduce the risk of either of the two primary composite outcomes (death or a cardiovascular event or death or a renal event) but was associated with an increased risk of stroke.7 These results were in line with several other studies,8, 9, 10 and based on this evidence, the Kidney Disease: Improving Global Outcomes (KDIGO) guideline group recommended that in adult patients, ESAs must not be used to intentionally increase hemoglobin above 13 g/dL (graded evidence level 1A).11 Interestingly in the “Normal Hematocrit Trial” by Besarab et al, patients that actually reached the target hematocrit of 40% had by far the lowest mortality of all participants.10 While this clearly could be due to a selection bias, the question remains, what if in these individuals, a normalization of hemoglobin could be superior to the guideline recommended partial correction approach. This has never been tested in a clinical trial and perhaps the full potential of ESAs is not exploited in a certain subgroup of patients with anemia and CKD/DKD.
Individualization or at least stratification of therapy based on patient characteristics is already part of some DKD guidelines. Metabolic control should preferentially be achieved by drugs selected based on the risk of associated hypoglycemia,12 and recent evidence suggests that some agents, like SGLT2 inhibitors or GLP1 agonists might even exert renal protection beyond their HbA1c lowering capacity. Interestingly their efficacy to reduce specific renal endpoints (eg, incidence of microalbuminuria) differs and thus in the future we could see an even more targeted administration.13, 14 However, additional efforts are necessary to maximize the risk‐benefit ratio in other areas of treatment of DKD. The recent guidelines of the American Diabetes Association state that multiple‐drug therapy is generally required to achieve blood pressure targets. However, the combination of an angiotensin converting enzyme (ACE) inhibitor and an angiotensin receptor blocker (ARB), and combinations of ACE inhibitors or ARBs with direct renin inhibitors should not be used.15 This recommendation is based on evidence that the risk of hyperkalaemia and/or acute kidney injury is increased with these combinations when compared to others.16 Nonetheless Palmer et al showed in the same meta‐analysis that double blockade of the renin‐angiotensin‐aldosterone system is the most efficient way to lower proteinuria and the risk of terminal end stage renal failure in patients with diabetes.16 Thus, if we could apply individualized medicine tools to identify the population at highest risk of side effects, an optimal approach of multiple‐drug antihypertensive and albuminuria lowering therapy in clinical practice could be individualized.
3. IMPLEMENTING PERSONALIZED MEDICINE: THE PATIENT'S PERSPECTIVE
Individualized medicine tries to incorporate all aspects of a person's disease and response to treatment and identify the treatment that will result in the optimal outcome for the individual patient. In order to achieve this, individualized medicine focuses on an individual's unique biological characteristics to tailor diagnostics and therapeutics to that specific patient, by utilizing biological ‐omics techniques, that is, genomics, proteomics, metabolomics, epigenomics and pharmacogenomics. In the past decade, a huge number of literature has been published on various ‐omics techniques, focusing on among others, mechanisms and pathways, biomarker discovery and better phenotyping. Indeed, systems biological approaches have led to the identification of critical molecular abnormalities in DKD and have directly led to development of new biomarkers and potential treatments for DKD.17 However, what is achieved in better clustering of patients is really just precise risk stratification. Importantly, risk stratification does not result in individualized medicine, but in more precise targeted medicine, or more precise defining of subgroups. Better risk stratification has been attempted in patients with diabetes, from risk engines like United Kingdom Prospective Diabetes Study (UKPDS),18 to better clustering of subgroups using sophisticated genotyping and bioinformatics studies.19
Nevertheless as important as it is to identify the various pathophysiology of DKD, other mechanisms in the person's life also play a role in disease progression. Just as different biological pathways lead to different types of renal disease in patients with diabetes, different social and individual aspects can affect a person's disease progression.20 In order to go from precise individualized treatment to personalized treatment, another dimension needs to be added to the plethora of biological ‐omics techniques. Taking in account an individual's personality, coping mechanisms, preferences, values, goals, health beliefs, social support network, financial resources and unique life circumstances—personomics—will also affect how and when a given health condition will manifest in that person and how that condition will respond to treatment.21 Not every patient has the same values in life and people might make different choices in remarkably similar situations. Importantly as well, the environment and society the person is in, as well as the resources available, will also have important roles in disease progression.
3.1. A patient's viewpoint and concerns for personalized medicine
Engaging patients and educating patients in order to advance personalized medicine is crucial. The clinical and research community asks a lot from patients, namely time and accepting of risks in exchange for hope of better treatment options and outcomes. Patients often say that they want hope and value innovation and exploration.22 Patients will and do give their time for research, with full awareness that they may not personally benefit from it. From a patient's perspective, there is often exciting news about new technology and the brave new world—but sometimes a yawning gap between the media promise and real life with kidney disease. People with renal disease are often enthused by the promise of a better life with better treatments; however, awareness and understanding of personalized medicine is variable.23 What people do want is personalized care that works for them as an individual and their individual condition, whether it is more effective drugs or wearable technology. However, translating research into clinical practice that actually reaches the patient is very slow, and researchers need to mitigate the balance between exploring current issues and difficulties of kidney disease against the uncertain future. Studies on how to best implement personalized therapy approaches should be prioritized in order to shorten the time gap between discovery and exploration studies and implementation.
There are many potential benefits for patients with personalized medicine, and patients have clear goals. Personalized medicine can have the ability to reduce the burden of disease, offer more convenience for patients, can possibly buy time, and could lead to cost savings. For example, using personalized medicine to better identify the right drug for the right patient can help minimize side effects. As patients and family members must take time to attend medical appointments, taking time off work or squeezing time away from their personal obligations can become burdensome. Furthermore, personalized medicine has the hope of earlier detection of disease and optimal treatment, or making a kidney transplant last longer, which can all lead to better outcomes and quality of life for patients and their families.
With the potential benefits of personal medicine come issues for patients as well. Personal privacy may become an area of concern. The genetic research and testing needed for personalized medicine reveals information that patients may not want to know or be disclosed. Researchers and clinicians need to treat patients' data with utmost care, and must be transparent on how data will be used to avoid public loss of trust. Furthermore, retaining realism and honesty on when and if personalized medicine tools will reach the patient, and their affordability needs to be addressed. Stakeholders in personalized medicine need to take a good look at patient‐research priorities and get better at explaining, following‐up and valuing patient input.
4. IMPLEMENTING PERSONALIZED MEDICINE: THE PAYER'S PERSPECTIVE
Personalized medicine encompasses therapies targeted at patients most likely to benefit. Providing these drugs requires regulatory approval granting marketing authorization but also (for healthcare systems funded by tax‐payers) evidence that these therapies work better or as well as existing therapies, and reflect good value for money. These health technology assessments (HTA) are performed by a decision body addressing paying for new therapies. For example, in England, the National Institute of Health and Care Excellence (NICE) serves this role for the NHS. Through its technology appraisal program, NICE asks two questions of manufacturers of drugs, devices and other health interventions: “how well does the new therapy work compared with what the NHS already offers?” and “do the costs of the new therapy reflect good value?” The NHS recognizes that, because it uses limited resources, it must provide care that brings the greatest benefit to the most people.
Often before a drug receives marketing authorization, and always before appraisal, HTA defines the decision problem, that is, the population, intervention (the technology of interest), comparator(s) (standard care) and outcomes. Ideally, for personalized medicine, the population will be limited to subgroup of people most likely to benefit. In estimating clinical effectiveness, HTA does not use outcome measures clinicians’ use in everyday care; for example, HTA would not attempt to compare the value of improving glomerular filtration with improving forced vital capacity. Appreciating that the same money cannot—at the same time—be spent on both, generic measures of effectiveness common to all diseases are used, such as the quality‐adjusted life year. Therapy is deemed “effective” if it makes people live longer and/or better. In calculating costs, the technology's price, but also the costs of administering it, its adverse effects, and any downstream complications are taken into account. For example, an appraisal of a drug that delays dialysis would account for the price of treatment, but also the cost of dialysis.
Appraising precision medicines includes challenges. Drugs offered to people most likely to benefit are also more likely to be clinically and cost‐effective. Yet, defining these patients from clinical trial data after‐the‐fact may not provide enough patients to find a drug's true effect, and may generate chance (incorrect) findings. Clinical trial strategies are evolving to identify which patients will benefit from the drug and who will have unwanted side effects. For example, the Study Of Diabetic Nephropathy With Atrasentan (SONAR) study (NCT01858532) uses an enrichment strategy with companion biomarkers to identify patients who respond favorably and unfavorably to the study medication. SONAR was initiated to characterize the long‐term renal effects of atrasentan 0.75 mg/day in patients with type 2 diabetes and nephropathy on top of standard care with an ACEi or ARB, plus diuretic therapy.24, 25 Eligible patients proceed to a 6‐week enrichment period, after which patients with a response in albuminuria (>30% reduction) and without unacceptable rise in body weight (<3 kg) or B‐type natriuretic peptide (<300 pg/mL) are randomly assigned to long‐term treatment with atrasentan or placebo, on top of ACEi or ARB, plus diuretic therapy. However, false positive findings may occur because subgroups are based on the biggest observed effects. Appraisals must include the costs of diagnosing patients with novel methodologies and companion diagnostics that are required in personalized medicine (ie, various ‐omics techniques). Setting prices and reimbursing for companion diagnostics need to be addressed. Different countries will have different appraisal methods and pricing and reimbursement policies.
5. IMPLEMENTING PERSONALIZED MEDICINE: THE GLOBAL PERSPECTIVE
Implementation of personalized medicine on a global scale includes a number of challenges, opportunities and current realities in clinical and research communities. There are huge variations in genetics, prevalence, rates of progression and outcomes in different regions of the world. Differences in socioeconomic status, and access to care also add to the challenges. There is an increasing appreciation that the interaction of genes and environment needs to be studied in the context of multiple genes and multiple environments, over time and space, in order to better understand these variabilities, and then address them to improve kidney health. The Global Burden of Disease and the International Society of Nephrology (ISN) Global Kidney Health Atlas, offer some insights into the impact of CKD on various populations. The latter describes the state of kidney care according to the six dimensions of Universal Health Coverage, using robust survey methodology, and capturing data from 125 countries, representing 93% of the world's population. The global distribution of nephrologists is described as 8.83 per 1.87 million population, with huge disparities between LIC, LMIC, upper‐middle‐income countries (UMIC) and high‐income countries (HIC). In a number of regions, no aspect of kidney care is funded, and registries are lacking for most kidney diseases or conditions throughout the world. Basic testing for urine and blood with reliable laboratory systems is not available in all locations, and the capacity for the conduct of longitudinal cohort studies, with bio‐sample banking, or clinical trials is disproportionately available to HIC, than LIC, with huge affected populations. Thus, the variation in access to care, research and knowledge translation currently limits the global community's ability to reap the rewards offered from personalized medicine.
The requirements and needs for personalized medicine include standardized clinical and hospital data systems. In addition, there is need to address ethical, social and legal issues associated with discoveries which may impact specific populations, or individuals, and to identify and contain costs related to data generated from molecular and genetic probing and drugs developed. In addition it will be important to address knowledge gaps of health care providers and patients about molecular genetics and biochemistry, interpretation of test results, and the relevance of information to treatment and prevention, so that the promise that personalized medicine (improved clinical outcomes, lower costs by managing existing diseases, increased therapeutic selection and medical adherence) can be achieved. In the longer term, earlier detection, curative interventions and reduction in disease burden, are promised.
In order to move forward with personalized medicine on a global scale, strategies to overcome challenges need to be identified. Defining new methods of conducting trials, collecting and storing specimens and sharing data is imperative in order to actualize the potential of personalized medicine in ways to reach patients. There are numerous complex interactions required to actualize the value of personalized medicine, and the need to manage expectations of patients, clinicians, clinical trialists, regulators, pharma development and funders. There are an increasing number of international consortia of networks which include patients, researchers and policy makers in different roles, so that we may begin to address some of the issues limiting our ability to move forward: the ISN is supporting the International Network of CKD (iNET CKD) cohorts, the ISN‐Advancing Clinical Trials (ISN‐ACT) is working to leverage existing infrastructures within ISN, and the European Association for the Study of Diabetes (EASD) European Diabetes Forum aims to address the full landscape of diabetes research and clinical care. Institutions such as the George Global Health Clinical Institute, and consortia such as Nephrotic Syndrome Study Network (NEPTUNE), Kidney Precision Medicine Project (KPMP) and Biomarker Enterprise to Attack Diabetic Kidney Disease (BEAt‐DKD) are all working on aspects of personalized medicine in kidney and/or diabetes. Additionally, the American Diabetes Association has given special focus to personalized medicine.26 Through collaborations, sharing of biosamples, databases, common protocols and multicenter trials conducted worldwide, an improved understanding of diseases affecting populations will be realized.
6. CONCLUSIONS
In this article, we highlighted different aspects for implementing personalized medicine in diabetes and CKD (Figure 1). Stratifying risk more accurately and more precise options for treatment need to be included into clinical practice guidelines. Treating the patient as an individual, with his or her own unique social and environment situations, is of great importance. The societal and environmental setting need to be considered, as culture, resources, access to healthcare and prevalence differ greatly around the globe. Appraising the cost effectiveness of precision medicines is necessary, as just the cost of new therapies but also the cost of diagnosis with novel methodologies and complications account. Implementing personalized medicine must incorporate all these aspects.
Figure 1.
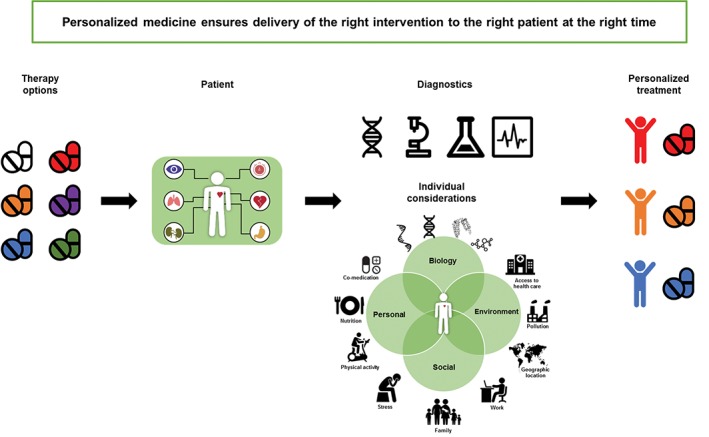
Implementing personalized medicine includes suitable therapy options for a single patient's‐specific illness and stage (co‐morbidities), using appropriate diagnostic tools and tailoring therapy to the patient's individual circumstances including underlying biology of the disease, environmental, social and personal factors (indicated by the green circles in the figure)
Healthcare delivery systems in their current form are economically unsustainable, given that healthcare consumes between 4% and 17% of the gross domestic product in Organisation for Economic Co‐operation and Development (OECD) countries.27 The infinite demand for care in aging populations, and finite clinical and financial resources, and an increasing high cost of chronic diseases are all drivers of this unsustainability. The promise of personalized medicine is that with deep phenotyping integrated with molecular profiles and clinical data on disease patterns, we will in the short term, improve clinical outcomes, control costs by managing existing diseases, enhance therapeutic selection and increase medical adherence. In the longer term, earlier detection, curative interventions and reduction in disease burden, are promised. In reality, personalized medicine has yet to show clinical, economic and social value, and the benefits and aspiration of personalized medicine have led to the recognizing of the complex reality of what we need to achieve the short‐ and longer‐term goals. Personalized medicine in diabetes and DKD is an ambitious goal, but this should not preclude aiming to bring patients the correct therapeutic strategies.
ACKNOWLEDGMENTS
The Precision Medicine Symposium which took place in December 2017 in Groningen was endorsed by the BEAt‐DKD project. The BEAt‐DKD project has received funding from the Innovative Medicines Initiative 2 Joint Undertaking under grant agreement No 115974. This Joint Undertaking receives support from the European Union's Horizon 2020 research and innovation programme and EFPIA.
Conflict of interest
All the authors declare no competing interests.
Author contribution
All authors participated in the writing, review and approval of this manuscript.
de Vries JK, Levin A, Loud F, Adler A, Mayer G, Pena MJ. Implementing personalized medicine in diabetic kidney disease: Stakeholders' perspectives. Diabetes Obes Metab. 2018;20(Suppl. 3):24–29. 10.1111/dom.13412
Funding information Innovative Medicines Initiative, Grant/Award Number: BEAt‐DKD 115974
REFERENCES
- 1. Rawshani A, Rawshani A, Franzen S, et al. Mortality and cardiovascular disease in type 1 and type 2 diabetes. N Engl J Med. 2017;376:1407‐1418. [DOI] [PubMed] [Google Scholar]
- 2. Atkins RC, Zimmet P. Diabetic kidney disease: act now or pay later. Nephrol Dial Transplant. 2010;25:331‐333. [DOI] [PubMed] [Google Scholar]
- 3. U.S. Renal Data System USRDS 2013 Annual Data Report: Atlas of Chronic Kidney Disease and End‐Stage Renal Disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2013:7.6.1; 7.9.1; 7.20.1. [Google Scholar]
- 4. Gordois A, Scuffham P, Shearer A, Oglesby A. The health care costs of diabetic nephropathy in the United States and the United Kingdom. J Diabetes Complicat. 2004;18:18‐26. [DOI] [PubMed] [Google Scholar]
- 5. Petrykiv SI, Laverman GD, de Zeeuw D, Heerspink HJL. The albuminuria‐lowering response to dapagliflozin is variable and reproducible among individual patients. Diabetes Obes Metab. 2017;19:1363‐1370. [DOI] [PubMed] [Google Scholar]
- 6. Felix Kropelin T, de Zeeuw D, Holtkamp FA, Packham DK, L Heerspink HJ. Individual long‐term albuminuria exposure during angiotensin receptor blocker therapy is the optimal predictor for renal outcome. Nephrol Dial Transplant. 2016;31:1471‐1477. [DOI] [PubMed] [Google Scholar]
- 7. Pfeffer MA, Burdmann EA, Chen CY, et al. A trial of darbepoetin alfa in type 2 diabetes and chronic kidney disease. N Engl J Med. 2009;361:2019‐2032. [DOI] [PubMed] [Google Scholar]
- 8. Singh AK, Szczech L, Tang KL, et al. Correction of anemia with epoetin alfa in chronic kidney disease. N Engl J Med. 2006;355:2085‐2098. [DOI] [PubMed] [Google Scholar]
- 9. Drueke TB, Locatelli F, Clyne N, et al. Normalization of hemoglobin level in patients with chronic kidney disease and anemia. N Engl J Med. 2006;355:2071‐2084. [DOI] [PubMed] [Google Scholar]
- 10. Besarab A, Bolton WK, Browne JK, et al. The effects of normal as compared with low hematocrit values in patients with cardiac disease who are receiving hemodialysis and epoetin. N Engl J Med. 1998;339:584‐590. [DOI] [PubMed] [Google Scholar]
- 11. KDIGO Clinical Practice Guideline for Anemia in Chronic Kidney Disease. Kidney Int Suppl. 2012;2:279‐335. [Google Scholar]
- 12. Guideline Development Group . Clinical Practice Guideline on management of patients with diabetes and chronic kidney disease stage 3b or higher (eGFR <45 mL/min). Nephrol Dial Transplant. 2015;30(suppl 2):ii1‐ii142. [DOI] [PubMed] [Google Scholar]
- 13. Wanner C, Inzucchi SE, Lachin JM, et al. Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med. 2016;375:323‐334. [DOI] [PubMed] [Google Scholar]
- 14. Mann JFE, Orsted DD, Brown‐Frandsen K, et al. Liraglutide and renal outcomes in type 2 diabetes. N Engl J Med. 2017;377:839‐848. [DOI] [PubMed] [Google Scholar]
- 15. American Diabetes Association . 9. Cardiovascular disease and risk management standards of medical care in diabetes‐2018. Diabetes Care. 2018;41:S86‐S104. [DOI] [PubMed] [Google Scholar]
- 16. Palmer SC, Mavridis D, Navarese E, et al. Comparative efficacy and safety of blood pressure‐lowering agents in adults with diabetes and kidney disease: a network meta‐analysis. Lancet. 2015;385:2047‐2056. [DOI] [PubMed] [Google Scholar]
- 17. Brosius FC, Ju W. The promise of systems biology for diabetic kidney disease. Adv Chronic Kidney Dis. 2018;25:202‐213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Stevens RJ, Kothari V, Adler AI, Stratton IM. United Kingdom Prospective Diabetes Study (UKPDS) group. The UKPDS risk engine: a model for the risk of coronary heart disease in type II diabetes (UKPDS 56). Clin Sci (Lond). 2001;101:671‐679. [PubMed] [Google Scholar]
- 19. Ahlqvist E, Storm P, Karajamaki A, et al. Novel subgroups of adult‐onset diabetes and their association with outcomes: a data‐driven cluster analysis of six variables. Lancet Diabetes Endocrinol. 2018;6:361‐369. [DOI] [PubMed] [Google Scholar]
- 20. Allen L, Williams J, Townsend N, et al. Socioeconomic status and non‐communicable disease behavioural risk factors in low‐income and lower‐middle‐income countries: a systematic review. Lancet Glob Health. 2017;5:e277‐e289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ziegelstein RC. Personomics. JAMA Intern Med. 2015;175:888‐889. [DOI] [PubMed] [Google Scholar]
- 22.European Alliance for Personalised Medicine. Report from Irish Presidency Conference. Innovation and Patient Access to Personalised Medicine. http://euapm.eu/pdf/EAPM_REPORT_on_Innovation_and_Patient_Access_to_Personalised_Medicine.pdf. Accessed May 2, 2018.
- 23. Budin‐Ljosne I, Harris JR. Patient and interest organizations' views on personalized medicine: a qualitative study. BMC Med Ethics. 2016;17:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Heerspink HJL, Andress DL, Bakris G, et al. Rationale and protocol of the study of diabetic nephropathy with AtRasentan (SONAR) trial: a clinical trial design novel to diabetic nephropathy. Diabetes Obes Metab. 2018;20:1369‐1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Heerspink HJL, Andress DL, Bakris G, et al. Baseline characteristics and enrichment results from the SONAR trial. Diabetes Obes Metab. 2018. 10.1111/dom.13315 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rich SS, Cefalu WT. The impact of precision medicine in diabetes: a multidimensional perspective. Diabetes Care. 2016;39(11):1854‐1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. OECD . Health at a Glance 2017: OECD Indicators. Paris: OECD Publishing; 2017, 10.1787/health_glance-2017-en. [DOI] [Google Scholar]


