Table 1.
Photocycloaddition reaction of phenanthrene‐9‐carboxaldehyde (1 a) and 2,3‐dimethyl‐2‐butene in the presence of chiral Lewis acids 3.
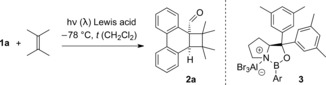
| Entry[a] |
λ
[a]
[nm] |
Lewis acid[b] |
Loading [mol %] |
t
[a]
[h] |
Yield[c]
[%] |
ee
[d]
[%] |
|---|---|---|---|---|---|---|
| 1 | 424 | 3 a | 50 | 14 | 53 | 56 |
| 2 | 435 | 3 a | 50 | 9 | 43 | 70 |
| 3 | 457 | 3 a | 50 | 11 | 49 | 79 |
| 4 | 457 | 3 b | 50 | 11 | 54 | 84 |
| 5 | 457 | 3 c | 50 | 11 | 62 | 97 |
| 6 | 457 | 3 c | 25 | 19 | 79 | 94 |
| 7 | 457 | 3 c | 10 | 26 | 81 | 94 |
[a] The reaction was performed at −78 °C with a substrate concentration of c=20 mm in CH2Cl2 at the indicated wavelength (λ) and for the indicated period of time (t). The olefin was used in excess (30 equiv). [b] 3 a: Ar=2,4,6‐trifluorophenyl; 3 b: Ar=2‐(trifluoromethyl)phenyl; 3 c: Ar=2,6‐dimethylphenyl. [c] Yield of isolated product. [d] The enantiomeric excess (ee) was determined by chiral‐phase HPLC analysis.
