Figure 2.
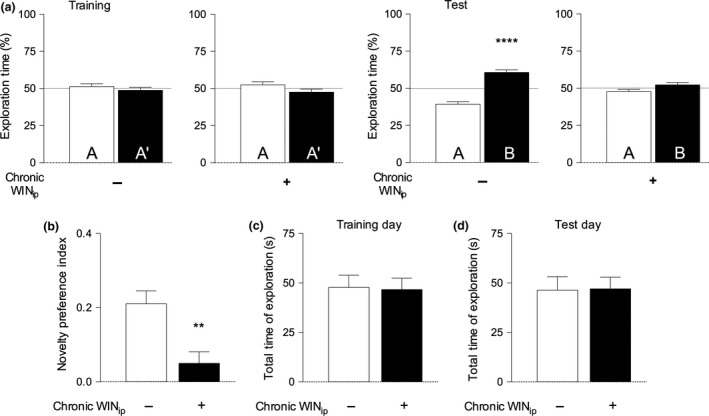
Chronic, intermittent WIN 55, 212‐2 treatment impairs recognition memory in the novel object recognition test (NORT). Panel a. Data are represented as % of exploration time. A denotes familiar object 1, A’ denotes familiar object 2, B denotes the novel object. – On the training day the % of time spent exploring each of the familiar objects did not differ between control and WIN 55,212‐2‐treated animals (p > 0.05, paired Student's t‐test). On the test day, control animals explored the novel object significantly more than the familiar object (****p < 0.0001, paired Student's t‐test). However, animals treated chronically with WIN 55,212‐2 spent a similar amount of time exploring both the familiar and novel object, (p > 0.05, paired Student's t‐test, comparing the % of time spent with a and b). Panel (b) – The Novelty Preference Index (NPI) is significantly decreased in WIN 55‐212‐2‐treated animals as compared with controls (**p < 0.01, unpaired Student's t‐test). A novelty index of zero represents absence of discrimination between objects (see Methods for details). The NPI of WIN55,212‐2‐treated mice was not significantly different from zero (p > 0.05, unpaired Student's t‐test), whereas in control mice the NPI was significantly different (p < 0.0001) from zero. Panels (c) and (d) – Total time of object exploration (TTE) during the training and test phase of novel object recognition test (NORT) was not significantly different between VEH and WIN‐treated animals (p > 0.05, unpaired Student's t‐test). An a posteriori Power Analysis for novel object recognition test (NORT) data was performed using G*power 3.1 software; considering a mean of 0.201 of preference for the novelty in control and 0.050 for drug‐treated animals (pooled SD (0.15), there is a 87.5% correct chance of correctly rejecting the null hypothesis (no differences on the t‐test), using 19 animals in the control group and 20 animals in the experimental group from a total of 39 animals.
