Figure 2.
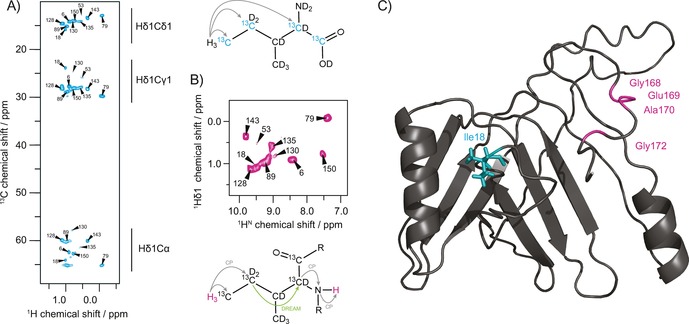
Assignment of isoleucine Cδ1 methyl groups and location of the protein−protein interface. A) 2D hCH correlation spectrum of isoleucine Cδ1 methyl labeled gp17.1 at 40 kHz MAS and 900 MHz external magnetic field. 2‐Ketobutyric acid‐13C4‐3,3‐d2 was used as a precursor resulting in the labeling of isoleucine residues as shown. A 6 ms hC cross‐polarization time enabled magnetization transfer from the Hδ1 methyl protons throughout the side‐chain and back. B) 2D HccanH spectrum to correlate methyl Hδ1 protons with their backbone amide protons for assignment. Magnetization is initially transferred from Hδ1 to Cδ1 and Cγ1 via CP, and subsequently to Cα via DREAM. Finally, magnetization transfer to HN is achieved by cαN and nH CP. C) Mapping of the determined tail tube interface onto the homology model of a polymerized gp17.1 subunit (taken from Langlois et al.31). The location of the Ile18 residue in the middle of the subunit suggests that the C‐terminus forms a molecular bridge that embraces the next monomer within the tail tube quaternary structure.
