Figure 1.
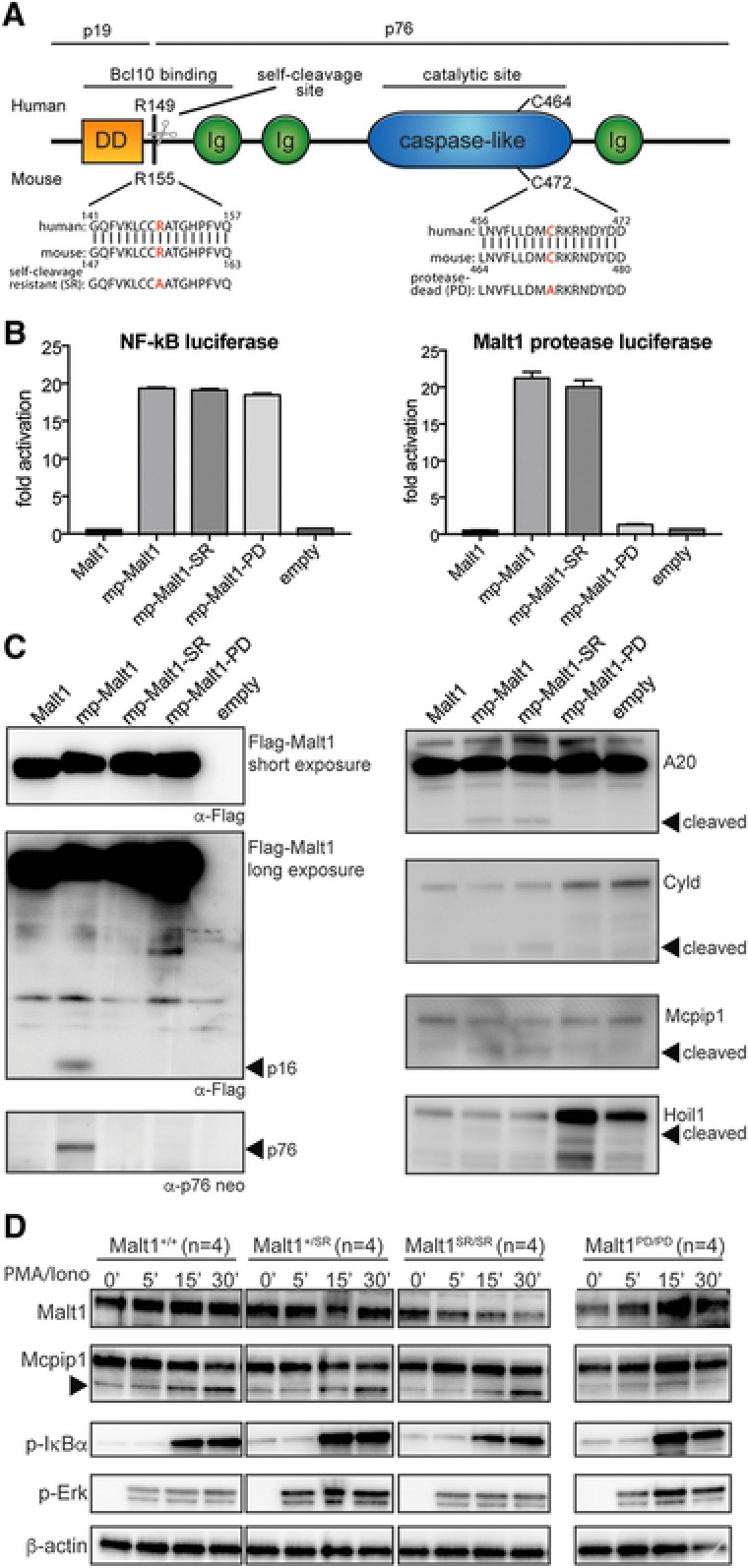
Malt1 R155A knock‐in mice express a catalytically active form of Malt1 but lack self‐cleavage activity (A) Schematic representation of Malt1 protein and its functional death domain (DD), immunoglobulin‐like domains (Ig), auto processing site and catalytic site. (B) MALT1 protease reporter assays of 293T‐BM cells transiently expressing a mouse wild‐type Malt1 (mp‐Malt1), the self‐cleavage resistant R155A mutant (mp‐Malt1‐SR), the protease‐death C472A mutant (mp‐Malt1‐PD) or empty vector (mock). Malt1 protease‐dependent luciferase activity is shown as fold induction of vector‐transfected cells. The data are shown as mean +SD of four pooled independent experiments, which were each performed in triplicates. (C) Cell lysates were immunoblotted with the indicated antibodies to detect MALT1, its N‐ and C‐terminal auto‐cleavage fragments (p16 and p76 respectively) and its proteolytic targets. Arrows indicate the cleavage fragments generated by Malt1 protease activity. (D) Immunoblot analysis of spleen of Malt1+/+, Malt1+/SR, Malt1SR/SR and Malt1PD/PD mice. Splenocytes from 4 mice were combined and then incubated with or without PMA and ionomycin for the indicated times. A non‐specific band generated with the Mcpip1 antibody was used a loading control. Data are representative of three independent experiments.
