Background/Objective
In this study we explored the neuroimaging characteristics of persons with specific small animal (cockroach) phobia to determine whether there are differences in cerebral activity between persons with and without cockroach phobia under conditions of phobic and non-phobic stimulation. Method: 24 adult persons (12 with phobia) were studied. The diagnosis of phobia was obtained with a structured interview and questionnaires. All participants were exposed to a 3D video presentation during an fMRI session. Results: The phobic group showed significant differential activations that were congruent with a dual route model of fear processing through the thalamus-amygdala (route I) and the thalamus-sensory and association cortex-entorhinal cortex-hippocampus-subiculum-amygdala (route II). Apart from this dual route, we also found differential activations in the globus pallidum, parahippocampal gyrus, insula, pars orbitalis, triangularis and opercularis of the frontal cortex, and cerebellum. Respect to non-phobic group, no activations were found in the insula or the anterior cingulate cortex. Conclusions: There seems to be a dual route depending on how persons with phobia to cockroaches process phobic stimuli. This double processing can have implications for the psychological treatment of specific phobias.
Keywords: Specific phobia, Cockroaches, Neuroimage, fMRI, Experimental study
Resumen
Antecedentes/Objetivo
En este estudio se exploran las características en neuroimagen de personas con fobia específica a pequeños animales (cucarachas), para determinar si existen diferencias en la actividad cerebral entre personas con y sin fobia a las cucarachas, bajo condiciones de estimulación fóbica y no fóbica. Método: Se estudiaron 24 adultos (12 con fobia). El diagnóstico de fobia específica se obtuvo mediante una entrevista estructurada y cuestionarios. Todos fueron expuestos a una presentación en video 3D durante una sesión de RMNf.
Resultados
El grupo con fobia mostró activaciones diferenciales significativas, que fueron congruentes con el modelo de doble ruta en el procesamiento del miedo, a través del tálamo-amígdala (ruta I), y tálamo-corteza entorrinal-hipocampo-subículo-amígdala (ruta II). Además, se encontraron activaciones diferenciales en el globo pálido, en el giro hipocampal, ínsula, y en los pars orbitalis, triangularis y opercularis. Con respecto al grupo control, no se observaron activaciones de la ínsula ni el cingulado.
Conclusiones
Parece evidenciarse un modelo de doble ruta en el procesamiento de estímulos fóbicos. Este doble proceso puede tener implicaciones para el tratamiento psicológico de las fobias específicas.
Palabras clave: Fobia específica, cucarachas, neuroimagen, RMNf, estudio experimental
Neuroimaging studies of patients with mental disorders have been conducted with the main aim of providing evidence of the neurological bases of such diseases. Such knowledge is expected to contribute to a better understanding of the neuropsychological mechanisms of psychopathology and consequently to the improvement of psychological and biological treatments (Anderson, Mizgalewicz, & Illes, 2013; Wright et al., 2013). Phobias are a type of anxiety disorder and refer to a high persistent anxiety response that people usually (but not always) consider excessive or irrational to the presence or anticipation of a threatening object or situation (American Psychiatric Association, APA, 2013). According to the APA, three groups of phobias (i.e., agoraphobia, social phobia, and specific phobias) are the most prevalent mental disorders overall. Of these, specific phobias have the highest prevalence rates, which range from 7% to 12.1% (APA, 2013; Kessler, Petukhova, Sampson, Zaslavky, & Wittchen, 2012). Clemente et al. (2014) pointed out that small animal phobia is one of the most disabling phobias because there is a possibility of encountering the animal in everyday life. According to these authors, 40% of specific phobias are phobias of small animals.
As shown by systematic reviews (Del Casale et al., 2012, Linares et al., 2012) and meta-analyses (Fullana et al., 2016; Ipser, Singh, & Stein, 2013), neuroimaging studies of phobias have found a group of similar brain areas and circuits to be related to brain responses to phobic stimuli. The areas most often found are those associated with limbic and paralimbic structures. The amygdala (especially the left amygdala) is the brain structure that has been found to be most closely associated with specific phobia in several studies (Britton, Gold, Deckersbach, & Rauch, 2009; Caseras, Mataix-Cols et al., 2010; Dilger et al., 2003; Goossens, Schruers, Peeters, Griez, & Sunaert, 2007; Li et al., 2013; Lipka, Miltner, & Straube, 2011; Straube, Glauer, Dilger, Mentzel, & Miltner, 2006; Straube, Mentzel, & Miltner, 2006). However, other brain areas have also been found to be activated by phobic stimuli. Such is the case of the left insula, cingulate gyrus, medial prefrontal cortex, hippocampus, and orbitofrontal cortex (Goossens et al., 2007; Schienle, Schäfer, Stark, & Vaitl, 2009; Wendt, Lotze, Weike, Hosten, & Hamm, 2008; Wright, Martis, McMullin, Shin, & Rauch, 2003).
The differences between those studies illustrate the complexity of neurological processes in phobias. However, the differences found may also be due to the methodological characteristics of those studies. In fact, studies differ from one another in many ways. A few examples are the use of clinical or subclinical samples, the type of phobia, age, gender, the presence or absence of a control comparative group, and procedural differences. In this regard, not all studies used the same type of fMRI machine. Despite commercial differences, most of the studies used 1.5 T machines. Machines with higher spatial resolution such as the one used in this study–3 T–allow a better delimitation of the brain structures detected and may explain some slight differences found between studies. There may also be specific effects depending on whether participants are shown pictures or a video presentation.
Considering the above, a more refined meta-analysis was performed of studies including an fMRI procedure whose participants included persons with small animal phobia and a healthy control group. The left amygdala and insular cortex were the brain areas found to be associated with the presence of phobic stimuli. Other structures involved in phobic responses were the fusiform gyrus, the left dorsolateral prefrontal cortex, and the left cingulate cortex. Overall, this review found that the role of frontal areas seemed to be less stable than that of limbic areas (Peñate et al., 2017). Considering the findings of the above-mentioned review, the aim of the present study was to provide data about brain activation in individuals with specific phobia (i.e., cockroach phobia). To do so, we intended to compare a group of persons with cockroach phobia to a control group of individuals without phobia. The fMRI was performed while participants watched a 3D video presentation of the stimuli, cockroaches moving around, to enhance the sensation of presence. This study was designed with these methodological requirements in order to avoid the heterogeneity of data obtained in previous studies. Apart from taking into account patients’ treatment preferences (Cooper & Norcross, 2016), as Beutler, Someah, Kimpara, and Miller (2016) pointed out, a multi-method approach is needed to increase the accuracy in selecting the best psychological treatment. In this regard, knowing which nuclei of the brain are related to fear can be a predominant step to improve the strategies aimed at eliminating its pathological learning (Ávila & Fullana, 2016).
Method
Participants
The sample was composed of 24 adults, of whom 12 had cockroach phobia and 12 did not have any phobias. Each of these two groups included eight female participants and four male participants. The mean age of phobic individuals was 40.08 years (SD 11.96) and that of non-phobic individuals was 24.08 years (SD 7.73). There was a significant difference between both groups in age (F(1)= 15.15; p = .001). All participants were right handed. For phobic individuals, we applied the following inclusion criteria: Being an adult with this type of phobia; the phobia had to be a primary psychological disorder and not be explained by another health condition, including another phobia or panic attack. The remaining inclusion criteria were not receiving any treatment for cockroach phobia, being right handed, having normal vision, and not having any impediment for a magnetic resonance imaging session. Non-phobic participants were recruited in a similar way among the students of the University of La Laguna in Tenerife, Spain. In this case, subjects obtained course credit in exchange for their participation.
Instruments
In order to verify the diagnosis of phobia, we used the questions about phobias and panic disorder of the Composite International Diagnostic Interview (CIDI), Version 2.1 (Kessler & Üstün, 2004). The CIDI is a structured interview for major mental disorders according to the CIE-10 criteria (World Health Organization, WHO, 1992). For the purposes of this study, items/questions related to specific phobia, social phobia, agoraphobia, and panic attack were selected. In addition, to verify whether participants met the inclusion criteria, a semi-structured interview was conducted asking for each specific criterion. Participants diagnosed with specific small animal phobia were included (F40.218; WHO, 1992). The Hamilton Anxiety Scale (HAM-A) and S-R Inventory of Anxiousness were administered as a complementary diagnosis.
The HAM-A (Hamilton, 1959) is a rating scale for clinicians developed to measure the severity of a patient's anxiety. It assesses 14 areas with a 5-point scale. It is a well-established scale (Thompson, 2015) with adequate psychometric properties, especially inter-rater reliability (interclass correlation coefficients ranged from .74 to .96; Bruss, Gruenberg, Goldstein, & Barber, 1994). In phobic participants, a score of 14 or higher was required.
The S-R Inventory of Anxiousness (Endler, Hunt, & Rosenstein, 1962) is a 14-item inventory that assesses the most usual symptoms (physiological, cognitive, and behavioral) associated with the response to an anxious stimulus with a 5-point Likert scale. The evaluator points out the target phobic stimulus (in our case, cockroaches) prior to the participant's response. This instrument has high internal consistency (.95; Endler et al., 1962) and adequate convergent validity (Kameoka & Tanaka-Matsumi, 1981).
Hand preference was assessed with the Edinburgh Handedness Inventory (Oldfield, 1971).
The nuclear magnetic resonance device used was a GE 3.0 T Signa Excite HD. Given that anxious participants could drop out of the study if they felt distress in the magnetic resonance device, an ASSET method (with 35 images with ang= 50°, TE= 2.1 ms, TR = 150 ms, 32 × 32 matrix and 6 mm slice width) was chosen to reduce time in the machine. Functional images were recorded with Gradient Echo (TR= 2000 ms, TE= 30 ms, FA = 75°, FOV = 25.6, Dim. Image = 64 × 64 × 32, Dim. Voxel = 4 × 4x4 mm).
Design
A two-group experimental design was used (Montero & León, 2007): phobic versus non-phobic individuals. Both groups were exposed to two different conditions: phobic stimuli (cockroaches) and neutral stimuli (wooden balls). Given that we used passive stimulation, a block design was used to present the stimuli in the magnetic resonance device. Each participant was randomly presented with 16 blocks of cockroach images and 16 blocks of images of wooden balls. The duration of each block was 20 seconds. All images had an identical white background. Participants were exposed to phobic and neutral stimuli in a stereoscopic 3D video with VisualStim digital MRI compatible 3D glasses (graphics card: GeForce 8600GT).
Procedure
The study was conducted from April to July 2016. Phobic participants were recruited through various media (website, press, flyers, radio, TV, and newspapers), asking for persons with small animal phobia to participate in an fMRI research study. It was explained that after participating in the study, subjects would receive an eight-session psychological treatment for cockroach phobia. Once verifying participants met the inclusion criteria (through the interview and questionnaires), participants were asked to undergo the magnetic resonance test. Prior to that, they signed an informed consent protocol. All participants who met an initial diagnosis of specific phobia (according questionnaire scores), was corroborated by the semi-structure interview (only participants with no-removable metallic belongings, such as implants or devices, were excluded due its interference with fMRI analysis). Participant in non-phobic group were assessed with the same instruments in order to determine they did not meet the criteria for phobic disorder.
This study was approved by the Ethics Committee for Research and Animal Welfare of the University of La Laguna (ref. CEIBA2012-0033).
fMRI and data analysis
Brain images were analyzed with Statistical Parametric Mapping (SPM 12) software. Pre-processing procedures included realigning, co-registering, segmenting (with forward deformation fields), normalizing (structural images with a 1 × 1x1 mm voxel size and functional images with a 4 × 4x4 mm voxel size), and smoothing (Gaussian Kernel of 8 mm, FWHM). Images were rendered and adjusted to the standard brain template of the Montreal Neurological Institute (MNI). In addition, the Xjview 8.14 package was used for neuroanatomic identification of active brain structures.
As regards specific statistical analyses, a whole-brain study with hierarchized random effects was performed as a general linear model. Intra- and intergroup analyses were made by conducting t-test comparisons. Because there was a significant difference in age, the age variable was introduced as a vector in those analyses (as a covariate).
As a conservative strategy, the Family-Wise Error (p<.05 FWE corrected) correction was used to assume when brain activation reached statistical significance. However, non-corrected probabilities were admitted when they were congruent with the biological model of phobias (but never higher than .005, uncorrected). With a voxel size of 4 × 4 × 4 mm, activations equal to or higher than a cluster size of 5 were selected.
Results
In order to test for internal validity, preliminary ANOVAs were performed to compare the differences in anxiety and phobia scores between the phobic group (PhG) and the non-phobic control group (CG). In general anxiety (HAM-A scale), the PhG showed a mean (M) of 18.25, with a standard deviation (SD) of 10.18. The CG had a M = 0.67, and a SD = 1.37. The ANOVA yielded an F(1) = 35.15, which was statistically significant (p = .001). In the phobia score (S-R scale), the PhG had a M = 27.5 (SD = 7.88) and the CG had a M = 7.75 (SD = 5.88). This difference was also significant [F(1) = 48.42; p = .001]. These data show that the PhG had higher scores in anxiety and cockroach phobia than the CG.
After testing for internal validity, the analyses were divided into two groups: First, we compared the brain activation of both the PhG and CG in the presence of phobic stimuli and neutral stimuli. Second, we compared the responses of the PhG and CG.
Phobic group
In this section we describe the results in brain activation obtained by the PhG when phobic stimulation and neutral stimulation were compared. Data are summarized in Table 1. These results correspond to the situation when phobic stimuli elicited a greater activation than neutral stimuli, because, as expected, participants did not show a greater activation in the presence of neutral stimuli compared to phobic stimuli.
Table 1.
Differences in functional brain activation in phobic participants. A greater activation was recorded in the presence of phobic stimuli, with FWE corrected and uncorrected p-value.
| Side | Coordinates | t value | p | Cohen's d* | |
|---|---|---|---|---|---|
| Thalamus | Right | 22, -28, -2 | 6.79 | .001 uncorr. | 2.90 |
| Thalamus | Left | −22, -28, -2 | 8.93 | <.0001 uncorr. | 3.81 |
| Pulvinar | Right | 21, -28, 2 | 6.15 | <.0001 uncorr. | 2.62 |
| Pulvinar | Left | −21, 28, 2 | 6.06 | <.0001 uncorr. | 2.07 |
| Amygdala | Right | 30, 0, -18 | 4.85 | .001 uncorr. | 2.01 |
| Amygdala | Left | −22, 0, -18 | 4.72 | .001 uncorr. | 3.15 |
| Superior occipital gyrus | Right | 26, -72, 30 | 7.38 | <.0001 FWE corr. | 2.55 |
| Superior occipital gyrus | Left | −22, -72, 34 | 5.99 | <.0001 FWE corr. | 5.19 |
| Inferior occipital gyrus | Left | −42, -76, -2 | 12.17 | <.0001 FWE corr. | 4.25 |
| Hippocampus | Left | −26, -28, -6 | 9.97 | <.0001 FWE corr. | 2.01 |
| Parahippocampal gyrus | Right | 34, -4, 22 | 4.71 | .001 uncorr. | 1.54 |
| Parahippocampal gyrus | Left | −34, -4, -22 | 3.62 | .001 uncorr. | 1.94 |
| Posterior cingulate gyrus | Right | 10, -44, 6 | 4.55 | .001 uncorr. | 1.55 |
| Posterior cingulate gyrus | Left | −10, -44, 6 | 3.63 | .001 uncorr. | 1.45 |
| Anterior cingulate gyrus | Left | −6, 36, 2 | 3.40 | .002 uncorr. | 3.48 |
| Superior parietal lobule | Left | −22, -64, 54 | 8.17 | <.0001 FWE corr. | 4.65 |
| Fusiform gyrus | Right | 42, -56, -18 | 10.90 | <.0001 FWE corr. | 3.45 |
| Fusiform gyrus | Right | 30, -84, 2 | 8.08 | <.0001 FWE corr. | 4.61 |
| Middle temporal gyrus | Right | 46, -68, 6 | 10.80 | <.0001 FWE corr. | 1.42 |
| Insula | Right | 46, 12, -2 | 3.34 | .002 uncorr. | 1.80 |
| Insula | Left | −34, 4, 18 | 4.22 | .001 uncorr. | 3.40 |
| Inferior frontal gyrus (p. triangularis) | Right | 54, 20, 22 | 7.97 | <.0001 FWE corr. | 3.13 |
| Inferior frontal gyrus (p. orbitalis) | Right | 46, 32, 2 | 7.34 | .002 FWE corr. | 2.75 |
| Inferior frontal gyrus (p. orbitalis) | Right | 30, 28, -10 | 6.46 | .015 FWE corr. | 2.81 |
| Inferior frontal gyrus (p. orbitalis) | Left | −26, 32, -2 | 6.58 | .015 FWE corr. | 2.64 |
| Inferior frontal g. (p. opercularis) | Right | 42, 8, 34 | 6.18 | .015 FWE corr. | 2.71 |
| Inferior temporal gyrus | Left | −42,-36, -18 | 6.35 | .015 FWE corr. | 2.70 |
| Precentral gyrus | Left | −50, 4, 42 | 6.34 | .004 FWE corr. | 2.59 |
| Posterior-medial frontal gyrus | Right | 6, 20, 62 | 6.07 | .015 FWE corr. | 2.80 |
| Globus Pallidum | Right | 18, -4, 2 | 6.57 | .007 FWE corr. | 2.59 |
| Pallidum | Left | −14, 0, 2 | 6.07 | .007 FWE corr. | 3.46 |
| Cerebellum (VI) | Left | −34,-52, -22 | 8.11 | <.0001 FWE corr. | 2.81 |
Note. Cohen's d was calculated taking into account t value and groups’ size.
As can be observed, differences in brain activation (i.e., phobic stimulation) were found in the thalamus, pulvinar nucleus, and amygdala; all areas were bilaterally activated). Differential activations were also detected in the superior occipital gyrus (bilateral), and inferior (left), left hippocampus, and parahippocampal gyrus (bilateral). Similar results were found for the cingulate gyrus: the posterior cingulate gyrus showed a bilateral activation but the anterior cingulate (and superior parietal) gyrus only showed activation on the left side.
Two areas were activated on the right side: the fusiform gyrus and middle temporal gyrus. The insula had bilateral activation. The inferior frontal gyrus had a differential activation depending on the brain side: its pars triangularis and opercularis were activated on the right side. By contrast, the pars orbitalis showed activation in two areas on the right side and in one area on the left side.
Finally, Table 1 shows the differences in both left inferior and left precentral circonvolutions, in the posterior part of the right medial frontal gyrus, in the globus pallidum, and in two areas of the left cerebellum.
Non-phobic group
The initial results were similar to those of the phobic group but affected fewer brain areas. As shown on Table 2, the thalamus, pulvinar nucleus, and amygdala were bilaterally activated by phobic stimuli. The inferior occipital gyrus (left), hippocampus (but only the right side), hippocampal gyrus (only left), and superior parietal lobule (only right) were also activated. The superior occipital gyrus was activated by phobic stimuli, but only on the right side and not bilaterally, as happened in the PhG; moreover, in the non-phobic group activation on the right side was observed in the inferior frontal gyrus (pars triangularis and opercularis) and fusiform gyrus. The precuneus was activated in this group but not in the phobic group.
Table 2.
Differences in functional brain activation in non-phobic participants. A greater activation was recorded in the presence of phobic stimuli, with FWE corrected and uncorrected p-value.
| Side | Coordinates | t value | p | Cohen's d | |
|---|---|---|---|---|---|
| Thalamus | Right | 22, 28, -2 | 6.22 | .001 uncorr. | 2.65 |
| Thalamus | Left | −22, -28, -2 | 8.32 | <.0001 uncorr. | 3.55 |
| Pulvinar nucleus | Right | 21, -28, 2 | 4.97 | <.0001 uncorr. | 2.12 |
| Pulvinar nucleus | Left | −21, 28, 2 | 5.51 | <.0001 uncorr. | 2.35 |
| Amygdala | Right | 30, 0, -18 | 3.55 | .001 uncorr. | 1.51 |
| Amygdala | Left | −26, 0, -18 | 5.28 | .001 uncorr. | 2.25 |
| Inferior occipital gyrus | Left | −42, -76, -2 | 16.87 | <.0001 FWE corr. | 7.19 |
| Superior occipital gyrus | Right | 26, -72, 30 | 7.66 | .05 FWE corr. | 3.27 |
| Hippocampus | Right | 26, -28, 6 | 10.45 | <.0001. FWE corr. | 4.46 |
| Parahippocampal gyrus | Left | −18,-28, -10 | 10.43 | .0001 FWE corr. | 4.45 |
| Superior parietal lobule | Left | −26, -64, 54 | 7.13 | <.0001 FWE corr. | 3.04 |
| Precuneus | Right | 14, -60, 62 | 8.26 | <.0001 FWE corr. | 3.52 |
| Fusiform gyrus | Right | 38, -52, -18 | 11.26 | <.0001 FWE corr. | 4.80 |
| Inferior frontal gyrus (p. triangularis) | Right | 54, 20, 22 | 8.94 | <.0001 FWE corr. | 3.81 |
| Inferior frontal gyrus (p. opercularis) | Right | 46, 8, 38 | 6.69 | .001 FWE corr. | 2.85 |
Differences between the phobic and non-phobic groups
Given that a significant difference was found in age between both groups, the age variable was introduced as a covariate. No statistically significant results were found regarding the modulating role of age in the following results. For all dependent variables, there were not F value higher than F(1,20) = 14.82 (coefficient from which age could be considered to play a significant role).
When both groups were compared in the phobic stimuli condition (see Table 3), the PhG showed a significantly higher activation in left side structures such as the thalamus, anterior cingulate gyrus, insula, and inferior temporal gyrus. On the right side of the brain, a higher activation was observed in the parahippocampal gyrus, inferior frontal gyrus (pars orbitalis), and middle temporal gyrus. The non-phobic group showed a higher significant activation in the left middle temporal gyrus.
Table 3.
Differences in functional brain activation between the phobic and non-phobic groups under the phobic stimuli condition, with FWE corrected and uncorrected p-value.
| Phobic > Control | Side | Coordinates | t value | p | Cohen's d |
|---|---|---|---|---|---|
| Thalamus | Left | −14, -16, 18 | 3.18 | .005 uncorr. | 1.32 |
| Parahippocampal gyrus | Right | 26, -44, 2 | 3.80 | .009 FWE corr. | 1.62 |
| Anterior cingulate gyrus | Left | −6, 36, 2 | 3.18 | .003 uncorr. | 1.32 |
| Insula | Left | −26, 16, 14 | 3.95 | .007 FWE corr. | 1.08 |
| Inferior frontal gyrus (p. orbitalis) | Right | 30, 28, -10 | 3.01 | .001 uncorr. | 1.58 |
| Middle temporal gyrus | Right | 54, -56, 10 | 3.75 | .01 FWE corr. | 1.06 |
| Inferior temporal gyrus | Left | −58, -60, -2 | 3.70 | .009 FWE corr. | 1.62 |
| Control > Phobic | |||||
| Middle temporal gyrus | Left | −66, -28, -6 | 4.77 | .006 FWE corr. | 1.17 |
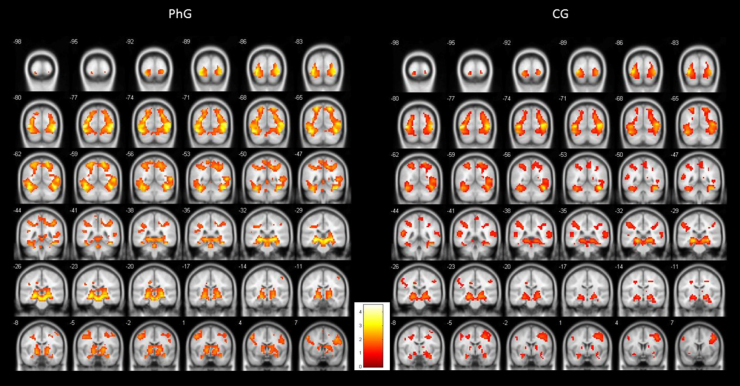
To illustrate the differential activations found between both groups, Figure 1 shows standardized brain activations during phobic stimulation for the phobic and non-phobic groups. As can be observed, both groups produced an emotional activity responding to phobic stimuli. But PhG attained higher brain activity (more yellow colour). This higher hemodynamic response can be observed, practically, in all brain slices. Also, it can be observed a more bilaterally activation in phobic individuals.
Figure 1.
Graphical representation of differential brain activations of phobic group (PhG) versus non-phobic control group (CG).
Discussion
In this study we attempted to answer two questions about brain activation in specific phobia. We intended to explore the differences under phobic stimulation vs. non-phobic stimulation and the differences between phobic and non-phobic individuals under phobic stimulation. In order to obtain a sample with greater homogeneity than that of previous studies, cockroach phobia was selected as the specific phobia. In addition, to increase the realism of stimuli, a 3D video stimuli presentation was used.
When we analyzed the brain responses of phobic individuals under phobic vs. neutral stimulation, we found a significant differential activation pattern that was consistent with previous data. The results obtained were congruent with a model of the existence of a two-route functional network in processing feared stimuli (Das et al., 2007, Granziera et al., 2011, Papez, 1995). According to this model, the information related to emotional stimuli follows a dual route toward the amygdala: (i) a “short and non-conscious route” that involves a direct link between the thalamus and the amygdala; and (ii) a “long and conscious route” that involves the thalamus-sensory and association cortex-entorhinal cortex-hippocampus-subiculum-amygdala. Both routes are used by emotional responses to phobic stimuli, with their behavioral, cognitive, physiological, and endocrine components.
In our study, individuals with phobia exhibited a differential bilateral activation of the thalamus that was greater on the left side and a differential activation of the amygdala (“short route”). In the “long route” we observed a differential activation of the superior and inferior occipital gyri (also detected by Del Casale et al., 2012). Lane, Chua, and Dolan (1999) found that these areas were highly sensitive to emotional visual information. Moreover, the intense activation of the hippocampus (bilaterally) indicates a certain level of perceived threat (Bach, 2015, Bach et al., 2014). The pulvinar portion is associated with the integration of sensory stimulation and attention. Along with the amygdala, the anterior cingulate gyrus is also involved in fear processing (with awareness in the long route and without awareness in the short route) and response modulation (Das et al., 2007). In phobic individuals, the anterior cingulate gyrus is activated on the left side, while the posterior cingulate gyrus is bilaterally activated. These data are consistent with those reported by Brewer, Garrison, and Whitfield-Gabrieli (2013), and Maddock, Garrett, and Buonocore (2003). By contrast, Fullana et al. (2016) found that the posterior cingulate gyrus was de-activated during differential conditioning.
Apart from this dual route, the bilateral activation of the globus pallidum (a structure involved in movement regulation, inhibiting cerebellum impulses) has been found in other studies on specific phobias (Fullana et al., 2016, Ipser et al., 2013). The bilateral activaction of the parahippocampal gyrus–a memory area particularly associated to the coding and recognition of enviromental stimuli–has been found in the studies reviewed by Fullana et al. (2016). The bilateral activation of the insula is associated with subjective distress in phobic individuals (Del Casale et al., 2012) and conditioned fear (Fullana et al., 2016).
The pars orbitalis, triangularis, and opercularis (inferior frontal cortex) exhibited a differential activation in the presence of phobic stimuli. These areas are involved in emotional regulation of phobias, generally according to the information received from the amygdala (Agustín-Pavón, 2013; Agustín-Pavón et al., 2012; Blackmon et al., 2011; Killgore et al., 2014; Sotres-Bayon, Cain, & Ledoux, 2006; Völlm et al., 2006; Vuilleumier & Pourtois, 2007). In addition, Bernal and Altman (2009) found that areas such as the inferior frontal gyrus, parietal lobule, temporal areas, and anterior cingulate gyrus are involved in the inhibition of both cognitive and motor responses. This may suggest that the phobic participants in our study were inhibiting cognitions and escape behaviors in the presence of phobic stimuli.
The cerebellum is a structure involved in the acquisition and expression of emotional associative learning that is directly related to fear behavior (Apps & Strata, 2015; Tovote, Fadok, & Lüthi, 2015). Our data are consistent with previous studies that found activation of the cerebellum in response to phobic stimulation (Caseras, Giampietro et al., 2010; Caseras, Mataix-Cols et al., 2010; Fullana et al., 2016; Ipser et al., 2013; Wendt et al., 2008).
When controls were exposed to phobic stimulation compared to neutral stimulation we also found activation in several similar brain areas, such as the thalamus, amygdala, occipital and parietal cortex, hippocampus, parahippocampal gyrus, fusiform gyrus, and supraorbital cortex. This result is similar to those reported by Caseras, Mataix-Cols et al. (2010). Yet, there were no differences in the insula and anterior cingulate gyrus. The prefrontal activation pattern was different compared to phobic individuals. There was activation in the pars triangularis and opercularis, but not in the pars orbitalis.
A possible explanation may be that non-phobic individuals were expressing emotional activity, but without the cognitive distress process (assigned to the left insular cortex) or the emotional regulation of the medial and orbital prefrontal cortex. Indeed, these participants did not show anxiety, as shown by their scores on the inventories. Other authors have found similar results, supporting the differences between anxiety (phobic individuals) and distress (non-phobic individuals). Wendt et al. (2008) studied individuals with spider phobia and suggested that the insula is related to a defensive response and the amygdala is related to the detection of emotionally relevant stimuli. Wright et al. (2003) also found higher activation of the amygdala to faces with emotional expressions than to phobic stimuli involving small animals.
These findings are consistent with our results, since seeing cockroaches caused emotional changes in both phobic and non-phobic individuals. Cockroaches as stimuli may be more likely to elicit disgust, aversion, and fear, because they are associated with the transmission of diseases, dirty places, sewers, etc. Yet, the phobic group also showed a defensive behavior, due to the activation of the insula. It would be interesting to test if this same pattern of activation can be found in individuals with specific phobia of small animals less associated with contamination and dirt (e.g., spiders or lizards).
Complementary results are provided by Stark et al. (2003), who found bilateral activation of the amygdala in response to unpleasant pictures, and joint activation of the amygdala and insula in response to phobic pictures. In our study, although cockroaches are insects that are likely to lead to a response of disgust and distress in non-phobic persons, the defensive response was only shown by phobic individuals.
In relation to the above, phobic individuals exhibited a higher activation of the right middle temporal gyrus than non-phobic individuals. However, controls showed higher activation of the left middle temporal gyrus. According to Schienle, Schäfer, Walter, Stark, and Vaitl (2005), this is consistent with our results, because activation of the right middle temporal gyrus is more associated with a phobic response, and activation of the left middle temporal gyrus is more associated with a disgust response.
This study has several limitations. First of all, sample size may affect the reliability of results (Button et al., 2013). This study only used a type of specific phobia (cockroach phobia) with few experimental stimulation conditions. We cannot establish if these results are due to a phobic condition or can also be observed in anxious non-phobic individuals. Moreover, we did not assess participants’ level of disgust as an emotional state different from fear/phobia (Bareither, Hasler, & Strasser, 2015). Also, due to sample size, gender differences could not been taken into account.
In conclusion, the data support the existence of a dual route in the processing of phobic stimuli: one route directly related to emotional processing (short/non-conscious), and another route involving several brain areas more related to a cognitive processing of feared stimuli (long/conscious).
Funding
This research was carried out thanks to the financial support provided by the Ministry of Science and Innovation of Spain (project PSI2013-42912-R).
References
- Agustín-Pavón C. El miedo en el cerebro. Mente y Cerebro. 2013;58:40–41. [Google Scholar]
- Agustín-Pavón C., Braesicke K., Shiba Y., Santangelo A.M., Mikheenko Y., Cockroft G., Asma F., Clarke H., Man M.S., Roberts A.C. Lesions of Ventrolateral Prefrontal or Anterior Orbitofrontal Cortex in Primates Heighten Negative Emotion. Biological Psychiatry. 2012;72:266–272. doi: 10.1016/j.biopsych.2012.03.007. [DOI] [PubMed] [Google Scholar]
- Anderson J.A., Mizgalewicz A., Illes J. Triangulating perspectives on functional neuroimaging for disorders of mental health. BMC Psychiatry. 2013;13:208. doi: 10.1186/1471-244X-13-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatry Association, APA . Fifth Edition. Author; Washington, DC: 2013. Diagnostic and Statistical Manual of Mental Disorders. [Google Scholar]
- Apps R., Strata P. Neuronal circuits for fear and anxiety - the missing link. Nature Reviews Neuroscience. 2015;16:642–643. doi: 10.1038/nrn4028. [DOI] [PubMed] [Google Scholar]
- Ávila A., Fullana M.A. El miedo en el cerebro humano. Mente y Cerebro. 2016;78:50–51. [Google Scholar]
- Bach D.R. El estudio del miedo. Mente y Cerebro. 2015;75:58–63. [Google Scholar]
- Bach D.R., Guitart-Masip M., Packard P.A., Miro J., Falip M., Fuentemilla L., Dolan R.J. Human Hippocampus Arbitrates Approach-Avoidance Conflict. Current Biology. 2014;24:541–547. doi: 10.1016/j.cub.2014.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bareither I., Hasler F., Strasser A. Nueve ideas para mejorar la neurociencia. Mente y Cerebro. 2015;73:43–51. [Google Scholar]
- Bernal B., Altman N. Neural networks of motor and cognitive inhibition are dissociated between brain hemispheres: an fMRI study. The International Journal of Neuroscience. 2009;119:1848–1880. doi: 10.1080/00207450802333029. [DOI] [PubMed] [Google Scholar]
- Beutler L.E., Someah K., Kimpara S., Miller K. Selecting the most appropriate treatment for each patient. International Journal of Clinical and Health Psychology. 2016;16:99–108. doi: 10.1016/j.ijchp.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackmon K., Barr W.B., Carlson C., Devinsky O., DuBois J., Pogash D., Thesen T. Structural evidence for involvement of a left amygdala-orbitofrontal network in subclinical anxiety. Psychiatry Research-Neuroimaging. 2011;194:296–303. doi: 10.1016/j.pscychresns.2011.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer J.A., Garrison K.A., Whitfield-Gabrieli S. What about the self is processed in the posterior cingulate cortex? Frontiers in Human Neuroscience. 2013;7:1–7. doi: 10.3389/fnhum.2013.00647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britton J.C., Gold A.L., Deckersbach T., Rauch S.L. Functional MRI study of specific animal phobia using an event-related emotional counting stroop paradigm. Depression and Anxiety. 2009;26:796–805. doi: 10.1002/da.20569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruss G.S., Gruenberg A.M., Goldstein R.D., Barber J.P. Hamilton anxiety rating scale interview guide: joint interview and test retest methods for interrater reliability. Psychiatry Research. 1994;53:191–202. doi: 10.1016/0165-1781(94)90110-4. PMID: 7824679. [DOI] [PubMed] [Google Scholar]
- Button K.S., Ioannidis P.A., Mokrysz C., Nosek B.A., Flint J., Robinson E.S.J., Munafò M.R. Power failure: Why small sample size undermines the reliability of neuroscience. Nature Reviews Neuroscience. 2013;14:365–376. doi: 10.1038/nrn3475. [DOI] [PubMed] [Google Scholar]
- Caseras X., Giampietro V., Lamas A., Brammer M., Vilarroya O., Carmona S., Rovira M., Torrubia R., Mataix-Cols D. The functional neuroanatomy of blood-injection-injury phobia: A comparison with spider phobics and healthy control. Psychological Medicine. 2010;40:125–134. doi: 10.1017/S0033291709005972. [DOI] [PubMed] [Google Scholar]
- Caseras X., Mataix-Cols D., Trasovares M.V., López-Solà M., Ortiz H., Pujol J., Soriano-Mas C., Giampietro V., Brammer M.J., Torrubia R. Dynamics of brain responses to phobic-related stimulation in specific phobia subtypes. Cognitive Neuroscience. 2010;32:1414–1422. doi: 10.1111/j.1460-9568.2010.07424.x. [DOI] [PubMed] [Google Scholar]
- Clemente M., Rey B., Rodríguez-Pujadas A., Bretón-López J., Barros-Loscertales A., Baños R.M., Botella C., Alcañiz M., Ávila C. A Functional Magnetic Resonance Imaging assessment of Small Animals’ Phobia Using Virtual Reality as a Stimulus. JMIR Serious Games. 2014;2:32–43. doi: 10.2196/games.2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper M., Norcross J.C. A brief, multidimensional measure of clients’ therapy preferences: The Cooper-Norcross Inventory of Preferences (C-NIP) International Journal of Clinical and Health Psychology. 2016;16:87–98. doi: 10.1016/j.ijchp.2015.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das P., Kemp A.H., Flynn G., Harris A.W.F., Liddell B.J., Whitford T.J., Peduto A., Gordon E., Williams L.M. Functional disconnections in the direct and indirect amygdala pathways for fear processing in schizophrenia. Schizophrenia Research. 2007;90:284–294. doi: 10.1016/j.schres.2006.11.023. [DOI] [PubMed] [Google Scholar]
- Del Casale A., Ferracuti S., Rapinesi C., Serata D., Piccirilli M., Savoja V., Kotzalidis G.D., Manfredi G., Angeletti G., Tatarelli R., Girardi P. Functional neuroimaging in specific phobia. Psychiatry Research: Neuroimaging. 2012;202:181–197. doi: 10.1016/j.pscychresns.2011.10.009. [DOI] [PubMed] [Google Scholar]
- Dilger S., Straube T., Mentzel H.J., Fitzek C., Reichenbach J.R., Hecht H., Miltner W.H.R. Brain activation to phobia-related pictures in spider phobic humans: An event-related functional magnetic resonance imaging study. Neuroscience Letters. 2003;348:29–32. doi: 10.1016/s0304-3940(03)00647-5. PMID: 12893418. [DOI] [PubMed] [Google Scholar]
- Endler N.S., Hunt J.McV., Rosenstein A.J. An S-R Inventory of Anxiousness. Psychological Monograph. 1962;76:143–146. [Google Scholar]
- Fullana M.A., Harrison B.J., Soriano-Mas C., Vervliet B., Cardoner N., Ávila-Parcet A., Radua J. Neural signatures of human fear conditioning: An updated and extended meta-analysis of fMRI studies. Mollecular Psychiatry. 2016;21:500–508. doi: 10.1038/mp.2015.88. [DOI] [PubMed] [Google Scholar]
- Goossens L., Schruers K., Peeters R., Griez E., Sunaert S. Visual presentation of phobic stimuli: Amygdala activation via an extrageniculostriate pathway? Psychiatry Research - Neuroimaging. 2007;155:113–120. doi: 10.1016/j.pscychresns.2006.12.005. [DOI] [PubMed] [Google Scholar]
- Granziera C., Hadjikhani N., Arzy S., Seeck M., Meuli R., Krueger G. In-vivo magnetic resonance imaging of the structural core of the Papez circuit in humans. Neuroreport. 2011;22:227–231. doi: 10.1097/WNR.0b013e328344f75f. [DOI] [PubMed] [Google Scholar]
- Hamilton M. The assessment of anxiety states by rating. British Journal of Medical Psychology. 1959;32:50–55. doi: 10.1111/j.2044-8341.1959.tb00467.x. [DOI] [PubMed] [Google Scholar]
- Ipser J.C., Singh L., Stein D.J. Meta-analysis of functional brain imaging in specific phobia. Psychiatry and Clinical Neurosciences. 2013;67:311–322. doi: 10.1111/pcn.12055. [DOI] [PubMed] [Google Scholar]
- Kameoka V.A., Tanaka-Matsumi J. The appropriateness of using the S-R Inventory of Anxiousness to measure sources of behavioral variability. Applied Psychological Measurement. 1981;5:229–235. [Google Scholar]
- Kessler R.C., Petukhova M., Sampson N.A., Zaslavsky A.M., Wittchen H.U. Twelve-month and lifetime prevalence and lifetime morbid risk of anxiety and mood disorders in the United States. International Journal of Methods in Psychiatric Research. 2012;21:169–184. doi: 10.1002/mpr.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler R.C., Üstün T.B. The World Mental Health (WMH) Survey Initiave Version of the World Health Organization, Composite International Diagnostic Interview (CIDI) International Journal of Methods in Psychiatric Research. 2004;13:93–121. doi: 10.1002/mpr.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killgore W.D.S., Britton J.C., Schwab Z.J., Price L.M., Weiner M.R., Gold A.L., Rosso I.M., Simon N.M., Pollack M.H., Rauch S.L. Cortico-limbic responses to masked affective faces across PTSD, panic disorder, and specific phobia. Depression and Anxiety. 2014;31:150–159. doi: 10.1002/da.22156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane R.D., Chua P.M.L., Dolan R.J. Common effects of emotional valence, arousal and attention on neural activation during visual processing of pictures. Neuropsychologia. 1999;37:989–997. doi: 10.1016/s0028-3932(99)00017-2. [DOI] [PubMed] [Google Scholar]
- Li H., Penzo M.A., Taniguchi H., Kopec C.D., Huang Z.J., Li B. Experience-dependent modification of a central amygdala fear circuit. Nature Neuroscience. 2013;16:332–341. doi: 10.1038/nn.3322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linares I.M.P., Trzesniak C., Chagas M.H.N., Hallak J.E.C., Nardi A.E., Crippa J.A.S. Neuroimaging in specific phobia disorder: A systematic review of the literature. Revista Brasileira de Psiquiatria. 2012;34:101–111. PMID: 22392396. [PubMed] [Google Scholar]
- Lipka J., Miltner W.H.R., Straube T. Vigilance for threat interacts with amygdala responses to subliminal threat cues in specific phobia. Biological Psychiatry. 2011;70:472–478. doi: 10.1016/j.biopsych.2011.04.005. [DOI] [PubMed] [Google Scholar]
- Maddock R.J., Garrett A.S., Buonocore M. Posterior cingulate cortex activation by emotional words: fMRI evidence from a valence decision task. Human Brain Mapping. 2003;18:30–41. doi: 10.1002/hbm.10075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montero I., León O. A guide for naming research studies in Psychology. International Journal of Clinical and Health Psychology. 2007;4:505–520. [Google Scholar]
- Oldfield R.C. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. PMID: 5146491. [DOI] [PubMed] [Google Scholar]
- Papez J.W. A proposed mechanism of emotion. 1937. The Journal of Neuropsychiatry and Clinical Neurosciences. 1995;7:103–112. doi: 10.1176/jnp.7.1.103. doi.org/7711480. [DOI] [PubMed] [Google Scholar]
- Peñate W., Fumero A., Viña C., Herrero M., Marrero R.J., Rivero F. A meta-analytic review of neuroimaging studies of specific phobia to small animals. European Journal of Psychiatry. 2017;31:23–36. [Google Scholar]
- Schienle A., Schäfer A., Stark R., Vaitl D. Long-term effects of cognitive behavior therapy on brain activation in spider phobia. Psychiatry Research-Neuroimaging. 2009;172:99–102. doi: 10.1016/j.pscychresns.2008.11.005. [DOI] [PubMed] [Google Scholar]
- Schienle A., Schäfer A., Walter B., Stark R., Vaitl D. Brain activation of spider phobics towards disorder-relevant, generally disgust -and fear- inducing pictures. Neuroscience Letters. 2005;388:1–6. doi: 10.1016/j.neulet.2005.06.025. [DOI] [PubMed] [Google Scholar]
- Sotres-Bayon F., Cain C.K., LeDoux J.E. Brain Mechanisms of Fear Extinction: Historical Perspectives on the Contribution of Prefrontal Cortex. Biological Psychiatry. 2006;60:329–336. doi: 10.1016/j.biopsych.2005.10.012. [DOI] [PubMed] [Google Scholar]
- Stark R., Schienle A., Walter B., Kirsch P., Sammer G., Ott U., Vaitl D. Hemodynamic responses to fear and disgust-inducing pictures: An fMRI study. International Journal of Psychophysiology. 2003;50:225–234. doi: 10.1016/s0167-8760(03)00169-7. [DOI] [PubMed] [Google Scholar]
- Straube T., Glauer M., Dilger S., Mentzel H.J., Miltner W.H.R. Effects of cognitive-behavioral therapy on brain activation in specific phobia. NeuroImage. 2006;29:125–135. doi: 10.1016/j.neuroimage.2005.07.007. [DOI] [PubMed] [Google Scholar]
- Straube T., Mentzel H.J., Miltner W.H.R. Neural mechanisms of automatic and direct processing of phobogenic stimuli in specific phobia. Biological Psychiatry. 2006;59:162–170. doi: 10.1016/j.biopsych.2005.06.013. [DOI] [PubMed] [Google Scholar]
- Thompson E. Hamilton Rating Scale for Anxiety (HAM-A) Occupational Medicine. 2015;65 doi: 10.1093/occmed/kqv054. 601-601. [DOI] [PubMed] [Google Scholar]
- Tovote P., Fadok J.P., Lüthi A. Neuronal circuits for fear and anxiety. Nature Reviews Neuroscience. 2015;16:317–331. doi: 10.1038/nrn3945. [DOI] [PubMed] [Google Scholar]
- Völlm B.A., Taylor A.N.W., Richardson P., Corcoran R., Stirling J., McKie S., Deakin J.F.W., Elliott R. Neuronal correlates of theory of mind and empathy: A functional magnetic resonance imaging study in a nonverbal task. NeuroImage. 2006;29:90–98. doi: 10.1016/j.neuroimage.2005.07.022. [DOI] [PubMed] [Google Scholar]
- Vuilleumier P., Pourtois G. Distributed and interactive brain mechanisms during emotion face perception: Evidence from functional neuroimaging. Neuropsychologia. 2007;45:174–194. doi: 10.1016/j.neuropsychologia.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Wendt J., Lotze M., Weike A.I., Hosten N., Hamm A.O. Brain activation and defensive response mobilization during sustained exposure to phobia-related and other affective pictures in spider phobia. Psychophysiology. 2008;45:205–215. doi: 10.1111/j.1469-8986.2007.00620.x. [DOI] [PubMed] [Google Scholar]
- World Health Organization, WHO . Author; Geneve: 1992. The ICD-10 classification of mental and behavioural disorders: Clinical descriptions and diagnostic guidelines. [Google Scholar]
- Wright B., Alderson-Day B., Prendergast G., Kennedy J., Bennett S., Docherty M., Whitton C., Manea L., Gouws A., Tomlinson H., Green G. Neural correlation of successful cognitive behaviour therapy for spider phobia: A magnetoencephalography study. Psychiatry Research-Neuroimaging. 2013;214:444–451. doi: 10.1016/j.pscychresns.2013.09.011. [DOI] [PubMed] [Google Scholar]
- Wright C.I., Martis B., McMullin K., Shin L.M., Rauch S.L. Amygdala and insular responses to emotionally valenced human faces in small animal specific phobia. Biological Psychiatry. 2003;54:1067–1076. doi: 10.1016/s0006-3223(03)00548-1. [DOI] [PubMed] [Google Scholar]