Table 1.
Phenylphenalenones isolated by HPLC and identified by 1D and 2D NMR spectroscopy and HRMS from ‘KTR’ leaves after infection with M. fijiensis strain E22
| No. | R t (min) | Compound | Chemical structure | Occurrence in Musa | |
|---|---|---|---|---|---|
| ‘Williams’ | ‘KTR’ | ||||
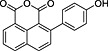
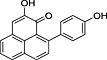
| 1 | 74.77 | 2‐(4′‐Hydroxyphenyl)‐1,8‐naphthalic anhydride |
|
+ | + |
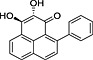
| 2 | 82.12 | trans‐2,3‐Dihydro‐2,3‐dihydroxy‐9‐phenylphenalenone |
|
+ | + |
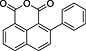
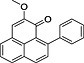
| 3 | 85.30 | 2‐Phenyl‐1,8‐naphthalic anhydride |
|
+ | + |
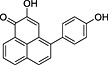
| 4 | 87.81 | 2‐Hydroxy‐4‐(4′‐hydroxyphenyl)‐1H‐phenalen‐1‐one (irenolone) |
|
+ | + |
| 5 | 89.35 | 2‐Hydroxy‐9‐(4′‐hydroxyphenyl)‐1H‐phenalen‐1‐one (hydroxyanigorufone) |
|
+ | + |
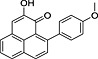
| 6 | 89.99 | 2‐Methoxy‐9‐(4′‐hydroxyphenyl)‐1H‐phenalen‐1‐one |
|
− | + |
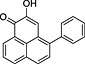
| 7 | 101.62 | Methoxyanigorufone |
|
− | + |
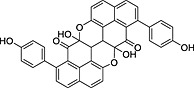
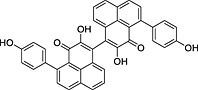
| 8 | 102.92 | Dihydroxyanigorootin |
|
+ | + |
| 9 | 104.20 | Anigorufone |
|
+ | + |
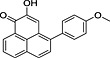
| 10 | 105.89 | 2‐Hydroxy‐9‐(4′‐methoxyphenyl)‐1H‐phenalen‐1‐one (4′‐O‐methylanigorufone) |
|
− | + |
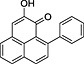
| 11 | 107.55 | Isoanigorufone |
|
− | + |
| 12 | 108.84 | 2‐Hydroxy‐4‐(4′‐methoxyphenyl)‐1H‐phenalen‐1‐one (4′‐O‐methylirenolone) |
|
+ | + |
| 13 | 118.98 | 3,3′‐bis‐Hydroxyanigorufone |
|
+ | + |
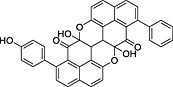
| 14 | 120.64 | Hydroxyanigorootin |
|
− | + |
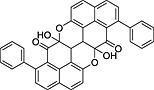
| 15 | 121.94 | Anigorootin |
|
+ | + |
Occurrence of the metabolites is represented by (+) or absence/not detectable (−).
HPLC, high‐performance liquid chromatography; KTR, Khai Thong Ruang; NMR, nuclear magnetic resonance; 1D, one‐dimensional; 2D, two‐dimensional.