Figure 5.
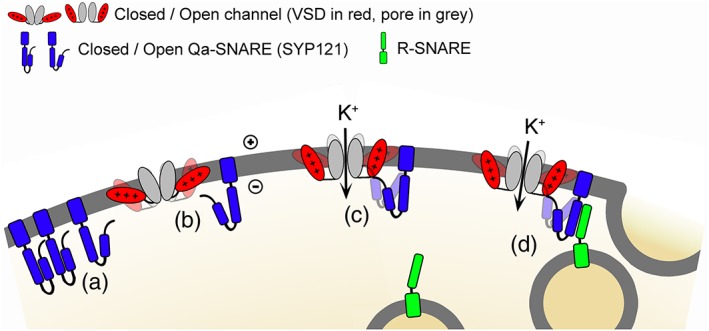
SYP121 binding promotes the open state of KAT1 for K+ influx and vesicle fusion. Schematic of the SNARE‐channel interactions deduced from previous analysis of SYP121 characteristics and from KAT1 gating kinetics described here: (a) SYP121 transits between the so‐called closed and open conformations that determine its availability for SNARE complex assembly, the open conformation also favouring channel binding. (b) Channel transitions between open and closed states (down and up conformations of the VSD, shown in red) are shown biased to the closed state, as indicated by the shading. (c) Once bound with SYP121, channel gating is biased to the open state. Transitions to the closed state incorporate a conformational “pull” on SYP121 (shading) and are thereby disfavoured. (d) Bound with the open channel, SYP121 is available for assembly with its cognate R‐SNARE and drives vesicle fusion. The cognate Qbc‐SNARE and other trafficking proteins omitted for clarity
