Abstract
Advanced microsurgical procedures are currently limited by human precision and manual dexterity. The potential of robotics in microsurgery is highlighted, including a general overview of applications of robotic assistance in microsurgery and its introduction in different surgical specialties. A new robotic platform especially designed for (super) microsurgery is presented. Results of an in vivo animal study underline its feasibility and encourage further development toward clinical studies. Future directions of robotic microsurgery are proposed.
Keywords: microsurgery, robotic surgery
1. POTENTIAL OF ROBOTICS IN MICROSURGERY
Extensive soft‐tissue defects following trauma or tumor resection are often reconstructed using microvascular free flaps. Microsurgery is regarded as one of the most technically demanding surgical disciplines.1 To perform microsurgical procedures a significant level of experience as well as the acquisition of challenging surgical skills is required since accuracy is essential to ensure high quality and outcome of the procedure. This accuracy is, however, limited by human capabilities. Robotic platforms offer potential advantages in the field of microsurgery.2 They are able to filter physiological tremor and allow for motion scaling (ie, translation of large movements into submillimetric movements), thereby improving surgical precision. Next, robotic platforms provide enhanced manipulation of instruments in tiny spaces that are hard to be visualized due to the location. Robotic assistance can also reduce issues related to human fatigue by offering enhanced dexterity for its user, the surgeon.
2. DA VINCI SYSTEM
Nowadays, microsurgery is characterized by the use of high magnification, fine instrumentation, and microsurgical skills.3 Currently, the da Vinci system is the most widely used robotic platform globally. The system offers a three‐dimensional stereoscopic vision, instruments with six degrees of motion freedom, scalable movements, and elimination of tremor. Despite these robotic advances, some experimental studies observed limitations when using this system for microsurgery.4, 5, 6, 7, 8 Nevertheless, microsurgical applications using the da Vinci system are increasingly being explored.9 The results of such efforts are not only contributing to establishing clinical evidence of the benefits of robotics in microsurgery, but also incrementing the interest of microsurgeons toward robotic assistance. This generates positive‐feedback for the expansion of research into a wider range of microsurgical applications in other surgical fields.
3. APPLICATIONS OF ROBOTIC MICROSURGERY
A general overview of applications of robotic assistance in microsurgery and its introduction in different surgical specialties is provided in the next section.
3.1. Cardiac surgery
Robotic assistance in microvascular surgery was initially introduced in cardiac surgery in 1998, when the first clinical computer‐enhanced arrested heart coronary artery bypass using the da Vinci system (Intuitive Surgical, Inc, Mountain View, CA) was performed.10 A few months later a similar procedure using the Zeus System (Computer Motion, Goleta, CA) was conducted.11 In 1999, an endoscopic coronary anastomosis on porcine heart models was performed using the da Vinci system.12 That year, the same group also successfully performed a totally endoscopic beating heart bypass operation using the Zeus System in a clinical setting.13
3.2. Transoral surgery and otolaryngology
Transoral robotic surgery with the da Vinci system has been performed in glottis microsurgery in a canine model.14 The platform has also been applied for pharyngeal and microlaryngeal dissections in cadaver models.15 In otology, new robot‐based microsurgical procedures were investigated for assistance in middle ear microsurgical procedures, such as stapedotomy.16
3.3. Ophthalmology
The application of robotic surgery in ophthalmology has not advanced at the same pace compared to other specialties. The latter is probably due to the relatively bulky instruments of the da Vinci system, which lack the finesse and specific design required in the field of ocular microsurgery.17 Robotic assistance has been evaluated in vivo in amniotic membrane transplant and pterygium surgery.18, 19 Robotics for penetrating keratoplasty has also shown to be feasible using the new Xi da Vinci system.17
3.4. Neurosurgery
Neurosurgical procedures using robotics include brachial plexus repair20 and sympathetic chain repair to treat Horner's syndrome21; both concern clinical cases.
3.5. Urology
In urology, robotic microsurgery has been widely applied to overcome technically demanding procedures, such as vasectomy reversal.22 Additionally, robotic assistance is becoming more‐and‐more common practice in male infertility procedures, eg, vasovasostomy and vasoepididymostomy.23 Techniques and outcomes of other common andrological microsurgical operations, such as spermatic cord denervation and testicular sperm extraction, have been reported as well.24
3.6. Plastic and reconstructive surgery
In 2005, the feasibility of the da Vinci system for robotic‐assisted microvascular anastomosis in a porcine free‐flap model was evaluated.25 In the next year, successful completion of an arterial anastomosis for a muscle sparing free transverse rectus abdominis myocutaneous (TRAM)‐flap using the aforementioned robotic platform was reported.26 In 2010, a report was published on the use of the da Vinci system for the reconstruction of oropharyngeal defects using a radial forearm, an anterolateral thigh flap, and a facial artery myomucosal flap.27 Another paper was published on the use of robotic assistance in latissimus dorsi muscle harvest.28 Rectus abdominis muscle harvest using robotic assistance has also been demonstrated.29, 30 In 2018, French surgeons reported the first case of nipple‐sparing mastectomy using the latest version of the da Vinci Xi surgical system.31
4. A NEW MASTER‐SLAVE PLATFORM FOR (SUPER) MICROSURGERY
Currently available robotic surgical platforms are not specifically designed for microsurgical procedures and therefore lack the specific requirements for microsurgery.8 Instruments are relatively bulky, with limited power and magnification of endoscopic cameras. To overcome these drawbacks, a new master‐slave platform, the MicroSure robot (MSR), was designed in 2014 by the Eindhoven University of Technology (TuE, Eindhoven, The Netherlands) in cooperation with the Maastricht University Medical Center (Maastricht, The Netherlands)32; a joint effort of technical engineers and microsurgeons.
4.1. The MSR
The first generation (Gen‐1) prototype of the MSR is composed of four subunits: a suspension ring, master manipulators, robotic arms, and foot pedals. The robotic arms are attached to the suspension ring and can be combined with genuine microsurgical instruments. The suspension ring can be attached to the operation table. The surgeon controls the master manipulators. The movements of the surgeon can be translated by means of motion scaling and tremor filtration to precise and controlled movements of the robotic arms. This way robotic precision can be combined with any delicate microsurgical instrument and any surgical microscope.
A more detailed description of the Gen‐1 MSR is provided in a recent publication in Plastic and Reconstructive Surgery.33 In the aforementioned report, the feasibility of performing microvascular anastomosis using the MSR on silicone vessels has been confirmed. Although the operation time of robotic‐assisted anastomosis was longer, a steep learning curve in time and quality was noted.
4.2. Evaluation of the MSR in an in vivo animal model
Following the promising results on silicone vessels, a feasibility study was conducted to investigate whether microvascular anastomosis in an in vivo rat model could successfully be performed using the Gen‐1 MSR.
4.2.1. Study design and procedures
Eight Wistar rats (Charles River Laboratories, Den Bosch, The Netherlands), 10 weeks of age and a minimum 300 g of weight, were used to perform microsurgical anastomosis. Ethical approval was obtained from the Maastricht University Institutional Review Board for Animal Use in Research (Maastricht, The Netherlands; project reference: DEC 2014‐078). A rat model was chosen because of the good resemblance of the abdominal aorta and the femoral artery compared with the vessels used in microsurgical free flaps and supermicrosurgical anastomosis, respectively, as performed in lymphaticovenous anastomosis.34 One microsurgeon (DB) performed all anastomoses. Time of completing the anastomosis and adverse events were recorded. All procedures were performed at the Central Animal Facilities of Maastricht University (Maastricht, The Netherlands).
The Gen‐1 MSR, as described above, was used. The slave arms were loaded with two genuine microsurgical needle holders (S&T AG Microsurgical Instruments, Neuhausen, Switzerland). The system was positioned over a previously dissected surgical field, and a genuine surgical microscope (Leica Wild M691 MEL53 Surgical Microscope; Leica Microsystems GmbH, Wetzlar, Germany) was placed in the center of the ring for magnification of the surgical field (Figures 1 and 2).
Figure 1.
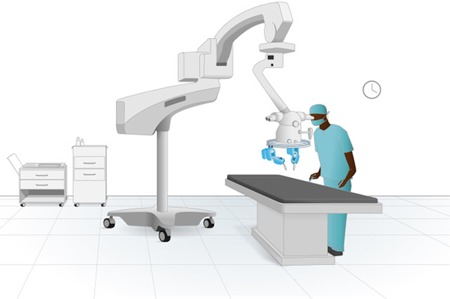
Schematic set‐up of the MicroSure robot. The system can be attached to a surgical microscope or to the operation table [Color figure can be viewed at wileyonlinelibrary.com]
Figure 2.
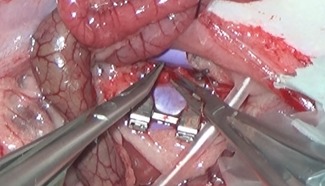
Preparation, transection, and anastomosis of the abdominal aorta using the first generation of the MicroSure robot [Color figure can be viewed at wileyonlinelibrary.com]
General anesthesia was initiated by a veterinarian, using 75 mg/kg ketamine and 0.5 mg/kg medetomidine intraperitoneal, and maintained by follow‐up injections of 35 mg/kg ketamine plus 0.25 mg/kg medetomidine. After completion and in vivo assessment of the anastomosis, the rat was killed with an overdose of pentobarbital (1:10, intraperitoneal).
4.2.2. Surgical procedure
4.2.2.1. Abdominal aorta
The abdomen of the rat was first shaved by the laboratory employee. The rat was carefully placed in the center of the microscope in a supine position. The surgeon performed a median laparotomy. The intestines were gently wrapped in wet gauzes and placed aside. The abdominal aorta was dissected from the renal arteries down to the aortic bifurcation. All other vessels that arise from the abdominal aorta were clipped using conventional small microsurgical clips (Ethicon, New Jersey, NJ). Afterwards, the abdominal aorta was secured with a double‐opposing microvascular clamp. The mean diameter of the aorta was 1.8 to 2.4 mm. The abdominal aorta was cut in the middle, and an end‐to‐end microvascular anastomosis was completed using Ethilon 10‐0 sutures (Ethicon).
4.2.2.2. Femoral artery
After shaving of the inguinal region of the rat by the laboratory employee, the rat was carefully placed in the center of the microscope. An incision was made in the inguinal region, the femoral artery and vein were dissected over a length of 1.5 cm. The femoral artery was secured with a double‐opposing microvascular clamp. The mean diameter of this artery was 0.7 to 0.8 mm. The femoral artery was cut in the middle, and an end‐to‐end microvascular anastomosis was completed using Ethilon 10‐0 sutures (Ethicon).
In both settings, the vessels were frequently rinsed with a solution of 500 cc sodium chloride and 25.000E/5 mL heparin (Heparin Leo; Leo Pharma BV, Amsterdam, The Netherlands). Frequently, a microvessel dilator was used for dilatation of the vessel.
4.2.3. Evaluation of the procedure
After completion of the anastomosis, the vascular clamp was removed, and the vessel was examined for any leakage or thrombosis. An extra suture was indicated by persistent leakage. Finally, the patency was checked with the “milking test” after 15 minutes.
After sacrificing the rat, the relevant vessel was excised and dissected and photographed for quality assessment.
Stages of completing the anastomosis were categorized into: preparation, piercing, knot‐tying, additional movements, and total surgical time. Preparation time started before picking up the needle and stopped after positioning the needle. Piercing time initiated before piercing the vessel and stopped after the needle fully pierced the second vessel. Knot‐tying time started before picking up the thread and stopped after finishing the third knot. Additional movements were defined as all the actions not related to suturing.
Descriptive statistics were calculated for time (mean and SD) for the robot and the conventional technique (SPSS Statistics for Windows, version 23.0; IBM Corp., Armonk, NY).
4.2.4. Findings
Seven anastomoses were performed using the MSR: three anastomoses of the abdominal aorta and four of the femoral artery. Two anastomoses were performed using the conventional microsurgical procedure to compare with the MSR procedure. Figure 3 shows two examples of an anastomosis.
Figure 3.
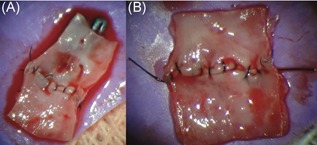
Femoral artery (A) and abdominal aorta (B) have been dissected after end‐to‐end anastomosis for quality assessment [Color figure can be viewed at wileyonlinelibrary.com]
Table 1 shows the mean surgical time of completing an anastomosis on either the abdominal aorta or femoral artery, respectively, by hand or by robotic assistance. The MSR required more time to perform an anastomosis on both aorta and femoral artery (mean, 69 [53‐87] and 27 [26‐29] minutes, respectively) as compared with the conventional technique (19 and 12 minutes, respectively).
Table 1.
Mean surgical time in minutes
| Preparation | Piercing | Knot‐tying | Additional movements | Total | |
|---|---|---|---|---|---|
| Aorta | |||||
| Hand, n = 1 | 3.7 | 2.3 | 3.6 | 6.8 | 19 |
| MSR, n = 3 | 12.6 | 8.8 | 21.4 | 18.7 | 69 (53‐87) a |
| Femoral artery | |||||
| Hand, n = 1 | 2.1 | 3.2 | 3.1 | 1.9 | 12 |
| MSR, n = 4 | 4.2 | 5.1 | 7.5 | 5.7 | 27 (26‐29) a |
Abbreviation: MSR, MicroSure robot.
Standard deviation.
All the performed anastomoses were patent except for one anastomosis performed with MSR on femoral artery. Two anastomoses showed leakage for which extra sutures were placed (hand, n = 1, 50%; MSR, n = 1, 14%). No adverse events were recorded during the hand anastomosis procedures. During the robotic procedures nine adverse events occurred, including a slave‐arm reset (n = 3), a system reset (n = 2), and a change of instruments (n = 3).
5. DISCUSSION
From a microsurgeon's perspective, the implementation of robotic assistance can facilitate enhanced precision and accuracy, which is crucial for more advanced (super) microsurgical procedures. To help overcome limitations of human capabilities, a new robotic platform was designed (the MSR), which is dedicated to microsurgery.
In a previous study on silicone vessels comparing robotic‐assisted anastomoses to the conventional technique, it was shown that completing microvascular anastomosis using the MSR was feasible but required significantly more time. Nevertheless, in the robotic‐assisted group, a rapid decline in anastomosis time and improvement of the quality of the anastomosis was found.33
The current animal study confirms the feasibility of performing a microvascular anastomosis on the abdominal aorta and femoral arteries in rats using the Gen‐1 MSR. In line with previous findings, the MSR still required more time performing the anastomoses as compared with the conventional microsurgical technique.
Several studies have reported rapidly progressing skills in inexperienced surgeons with robotic‐assisted microsurgery, thereby concluding that basic microsurgical skills are more easily mastered with robotic assistance compared with the conventional manual technique.35, 36
A limitation of this study was the small number of anastomoses created in a small number of animals. Nevertheless, this study was intended as a pilot study to investigate whether microvascular anastomoses on the abdominal aorta and femoral arteries in rats could successfully be performed using the Gen‐1 MSR.
This Gen‐1 prototype of the MSR has proven to be an effective instrument, which needs further development and improvement. To achieve better results, technicians have now produced a new prototype of the MSR (Gen‐2). The Gen‐2 is designed more ergonomically to minimize weariness and further improve manual dexterity.
6. FUTURE DIRECTIONS OF ROBOTIC MICROSURGERY
Future research projects will need to focus on further improvement of already available robotic platforms and the development of new robots for specific indications. Technological advances will allow microsurgeons to perform robotic‐assisted microsurgery with enhanced precision and will also make new procedures possible. The authors believe that a variety of factors will dictate the future of this revolution in surgery, in particular, microsurgery.
6.1. Microsurgical instruments
Microsurgical tools should be especially designed to meet the needs of the surgeons in terms of size and degree of articulation. Some microsurgical procedures demand specific microinstrumentations to reach difficult anatomical regions, eg, during reconstruction after resection of oropharyngeal tumors or during delicate ophthalmic procedures. The introduction of new instruments such as microDoppler probes, hydro‐jet dissectors will further evolve and augment the surgeon's capabilities. Incorporation of biosensors on instruments could facilitate future guided super‐microsurgery.
6.2. Haptic feedback
The lack of haptic feedback is frequently seen as a disadvantage of robotic surgery. In microsurgery, visual cues can be used to mimic the perception of haptic feedback, even when true haptic feedback is not present. The development and incorporation of haptic feedback in (super) microsurgery would allow microsurgeons to feel extremely small forces that occur. Such a theoretical advancement would improve surgical precision, tissue handling, and procedural outcome.
6.3. Image‐guided surgery
Optimal visualization of the surgical site is paramount during microsurgery. The development and incorporation of novel imaging modalities such as three‐dimensional imaging, high‐spectral imaging, and real‐time navigation systems provide promising opportunities for the continuous refinement of microsurgery. Implementation of intraoperative visualization modalities such as near‐infrared fluorescence (NIRF) imaging could facilitate real‐time intraoperative anatomical navigation and contribute to critical decision‐making.37, 38
Incorporation of intraoperative image‐guidance could also compensate for the lack of haptic feedback. For example, perioperative NIRF imaging after intravenous indocyanine green is considered to possess great advantages for the assessment of anastomosis viability and for monitoring of blood flow and tissue perfusion. Ultimately, such advances could predict the outcomes of reconstructive microsurgery.39, 40
6.4. Cognitive robots
Another potential advantage of using robotic platforms for microsurgical procedures is that any movement and force can be registered. Such knowledge can be applied to improve surgical technique, facilitate microsurgical training, and standardize procedural outcomes.
Cognitive robots refers to intelligent robotic systems with cognitive skills and the ability of “selflearning.” Such systems are supported by surgical data science and so‐called “big data analytics,” which enables semiautomated surgery. Online surgical data in combination with robot registration, calibration, and kinematic data can become a game changer in medicine.
In conclusion, the described animal study underlines the feasibility of performing a microvascular anastomosis in a rat model using a first prototype of a new robotic platform, which is specifically designed for microsurgery. Further development and studies are needed to evaluate performance and outcomes in the (pre)clinical setting. Robotic technology will propel microsurgery to higher levels, thereby enhancing quality and enabling new treatment possibilities.
CONFLICTS OF INTEREST
Tom van Mulken is the Chief M edical Officer and shareholder in the spin‐off company MicroSure, which also bears the costs of travel. Rene van der Hulst is a shareholder of MicroSure. Raimondo Cau is the Chief Technical Officer and shareholder of MicroSure. The other authors have no conflicts of interest.
SYNOPSIS
Potential of robotic surgery is addressed including an overview through different specialities. First results in an in vivo animal model of a new robotic platform especially designed for (super) microsurgery is presented.
van Mulken TJM, Schols RM, Qiu SS, et al. Robotic (super) microsurgery: Feasibility of a new master‐slave platform in an in vivo animal model and future directions. J Surg Oncol. 2018;118:826–831. 10.1002/jso.25195
References
REFERENCES
- 1. Clarke NS, Price J, Boyd T, et al. Robotic‐assisted microvascular surgery: skill acquisition in a rat model. J Robot Surg. 2018;12:331‐336. [doi]. 10.1007/s11701-017-0738-5 [DOI] [PubMed] [Google Scholar]
- 2. Selber J. Robotic surgery. J Reconstr Microsurg. 2012;28:433‐434. [DOI] [PubMed] [Google Scholar]
- 3. Park YM, Kim HR, Cho BC, Keum KC, Cho NH, Kim SH. Transoral robotic surgery‐based therapy in patients with stage III‐IV oropharyngeal squamous cell carcinoma. Oral Oncol. 2017;75:16‐21. [DOI] [PubMed] [Google Scholar]
- 4. Katz RD, Rosson GD, Taylor JA, Singh NK. Robotics in microsurgery: use of a surgical robot to perform a free flap in a pig. Microsurgery. 2005;25:566‐569. [DOI] [PubMed] [Google Scholar]
- 5. Katz RD, Taylor JA, Rosson GD, Brown PR, Singh NK. Robotics in plastic and reconstructive surgery: use of a telemanipulator slave robot to perform microvascular anastomoses. J Reconstr Microsurg. 2006;22:53‐57. [DOI] [PubMed] [Google Scholar]
- 6. Selber JC. Transoral robotic reconstruction of oropharyngeal defects: a case series. Plast Reconstr Surg. 2010;126:1978‐1987. [DOI] [PubMed] [Google Scholar]
- 7. Taleb C, Nectoux E, Liverneaux PA. Telemicrosurgery: a feasibility study in a rat model. Chir Main. 2008;27:104‐108. [DOI] [PubMed] [Google Scholar]
- 8. van der Hulst R, Sawor J, Bouvy N. Microvascular anastomosis: is there a role for robotic surgery? J Plast, Reconstr Aesthet Surg. 2007;60:101‐102. [DOI] [PubMed] [Google Scholar]
- 9. Ibrahim AE, Sarhane KA, Selber JC. New frontiers in robotic‐assisted microsurgical reconstruction. Clin Plast Surg. 2017;44:415‐423. [DOI] [PubMed] [Google Scholar]
- 10. Loulmet D, Carpentier A, D’attellis N, et al. Endoscopic coronary artery bypass grafting with the aid of robotic assisted instruments. J Thorac Cardiovasc Surg. 1999;118:4‐10. [DOI] [PubMed] [Google Scholar]
- 11. Reichenspurner H, Damiano RJ, Mack M, et al. Use of the voice‐controlled and computer‐assisted surgical system ZEUS for endoscopic coronary artery bypass grafting. J Thorac Cardiovasc Surg. 1999;118:11‐16. [DOI] [PubMed] [Google Scholar]
- 12. Boyd WD, Desai ND, Kiaii B, et al. A comparison of robot‐assisted versus manually constructed endoscopic coronary anastomosis. Ann Thorac Surg. 2000;70:839‐842. [DOI] [PubMed] [Google Scholar]
- 13. Boyd WD, Rayman R, Desai ND, et al. Closed‐chest coronary artery bypass grafting on the beating heart with the use of a computer‐enhanced surgical robotic system. J Thorac Cardiovasc Surg. 2000;120:807‐809. [DOI] [PubMed] [Google Scholar]
- 14. O’malley BW, Jr , Weinstein GS, Hockstein NG. Transoral robotic surgery (TORS): glottic microsurgery in a canine model. Journal Voice. 2006;20:263‐268. [DOI] [PubMed] [Google Scholar]
- 15. Hockstein NG, Nolan JP, O’Malley BW, Jr. , Woo YJ. Robot‐assisted pharyngeal and laryngeal microsurgery: results of robotic cadaver dissections. Laryngoscope. 2005;115:1003‐1008. [DOI] [PubMed] [Google Scholar]
- 16. Kazmitcheff G, Nguyen Y, Miroir M, et al. Middle‐ear microsurgery simulation to improve new robotic procedures. BioMed Res Int. 2014;2014:891742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chammas J, Sauer A, Pizzuto J, et al. Da Vinci Xi robot–assisted penetrating keratoplasty. Transl Vis Sci Technol. 2017;6:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bourcier T, Chammas J, Becmeur PH, et al. Robotically assisted pterygium surgery: first human case. Cornea. 2015;34:1329‐1330. [DOI] [PubMed] [Google Scholar]
- 19. Bourcier T, Becmeur PH, Mutter D. Robotically assisted amniotic membrane transplant surgery. JAMA Ophthalmol. 2015;133:213‐214. [DOI] [PubMed] [Google Scholar]
- 20. Dobbs TD, Cundy O, Samarendra H, Khan K, Whitaker IS. A systematic review of the role of robotics in plastic and reconstructive surgery‐from inception to the future. Front Surg. 2017;4:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Latif MJ, Park K, Razi SS, et al. Robotic microsurgical nerve grafting for sympathectomy reversal: technique and feasibility for first human case. Int J Med Robot Comput Assist Surg. 2011;7:27. [Google Scholar]
- 22. Tokas T, Gozen AS, Rassweiler J. Robotically assisted bilateral vasovasostomy, after a successful bilateral vasectomy. J Endourol. 2013;27:A309. [Google Scholar]
- 23. Dickey RM, Pastuszak AW, Hakky TS, Chandrashekar A, Ramasamy R, Lipshultz LI. The evolution of vasectomy reversal. Curr Urol Rep. 2015;16:40. [DOI] [PubMed] [Google Scholar]
- 24. Parekattil SJ, Gudeloglu A. Robotic assisted andrological surgery. Asian J Androl. 2012;15:67‐74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Katz RD, Rosson GD, Taylor JA, Singh NK. Robotics in microsurgery: use of a surgical robot to perform a free flap in a pig. Microsurgery. 2005;25:566‐569. [DOI] [PubMed] [Google Scholar]
- 26. van der Hulst R, Sawor J, Bouvy N. Microvascular anastomosis: is there a role for robotic surgery? J Plast, Reconstr Aesthet Surg. 2007;60:101‐102. [DOI] [PubMed] [Google Scholar]
- 27. Selber JC. Transoral robotic reconstruction of oropharyngeal defects: a case series. Plast Reconstr Surg. 2010;126:1978‐1987. [DOI] [PubMed] [Google Scholar]
- 28. Selber JC, Baumann DP, Holsinger FC. Robotic latissimus dorsi muscle harvest: a case series. Plast Reconstr Surg. 2012;129:1305‐1312. [DOI] [PubMed] [Google Scholar]
- 29. Patel N, Pedersen J. Robotic harvest of the rectus abdominis muscle: a preclinical investigation and case report. J Reconstr Microsurg. 2012;28:477‐480. [DOI] [PubMed] [Google Scholar]
- 30. Pedersen J, Song DH, Selber JC. Robotic, intraperitoneal harvest of the rectus abdominis muscle. Plast Reconstr Surg. 2014;134:1057‐1063. [DOI] [PubMed] [Google Scholar]
- 31. Sarfati B, Honart JF, Leymarie N, Rimareix F, Al Khashnam H, Kolb F. Robotic da Vinci Xi‐assisted nipple‐sparing mastectomy: first clinical report. Breast J. 2018;24:373‐376. [DOI] [PubMed] [Google Scholar]
- 32.Cau R. Design and Realization of a Master‐Slave System For Reconstructive Microsurgery [Thesis]. Eindhoven, The Netherlands: Eindhoven University of Technology, 2013; 154
- 33. van Mulken TJM, Boymans CAEM, Schols RM, et al. Preclinical experience using a new robotic system created for microsurgery. Plast Reconstr Surg. 2018. In press. [DOI] [PubMed] [Google Scholar]
- 34. Shurey S, Akelina Y, Legagneux J, Malzone G, Jiga L, Ghanem AM. The rat model in microsurgery education: classical exercises and new horizons. Arch Plast Surg. 2014;41:201‐208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kaul S, Shah NL, Menon M. Learning curve using robotic surgery. Curr Urol Rep. 2006;7:125‐129. [DOI] [PubMed] [Google Scholar]
- 36. Jacobs S, Holzhey D, Strauss G, Burgert O, Falk V. The impact of haptic learning in telemanipulator‐assisted surgery. Surg Laparosc Endosc Percutan Tech. 2007;17:402‐406. [DOI] [PubMed] [Google Scholar]
- 37. Schols RM, Connell NJ, Stassen LPS. Near‐infrared fluorescence imaging for real‐time intraoperative anatomical guidance in minimally invasive surgery: a systematic review of the literature. World J Surg. 2015;39:1069‐1079. [DOI] [PubMed] [Google Scholar]
- 38. Cornelissen AJM, van Mulken TJM, Graupner C, et al. Near‐infrared fluorescence image‐guidance in plastic surgery: a systematic review. Eur J Plast Surg. 2018;41:269‐278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mothes H, Donicke T, Friedel R, Simon M, Markgraf E, Bach O. Indocyanine‐green fluorescence video angiography used clinically to evaluate tissue perfusion in microsurgery. J Trauma. 2004;57:1018‐1024. [DOI] [PubMed] [Google Scholar]
- 40. Lee BT, Matsui A, Hutteman M, Lin SJ, Winer JH, Laurence RG, Frangioni JV. Intraoperative near‐infrared fluorescence imaging in perforator flap reconstruction: current research and early clinical experience. J Reconstr Microsurg. 2010;26:59‐65. [DOI] [PMC free article] [PubMed] [Google Scholar]


