Table 2.
Hydrogenation of cyclic carbonates using Mn complex 1 as a pre‐catalyst.[a,b]
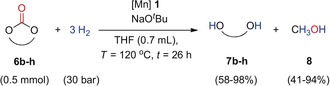
| Entry | Carbonate | [Mn] [mol %] |
Conv. [%] |
Diol [7, %] |
Methanol [8, %] |
|
|---|---|---|---|---|---|---|
| 1 |
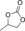
|
6 b | 1 | 89 | 75 | 66 |
| 2 | 2 | >99 | 82 | 75 | ||
| 3 |
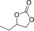
|
6 c | 1 | 86 | 82 | 65 |
| 4 | 2 | >99 | 97 | 74 | ||
| 5 |
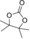
|
6 d | 1 | 82 | 76 | 66 |
| 6[c] |
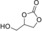
|
6 e | 1 | 67 | 59 | 43 |
| 7[c] | 2 | 77 | 71 | 60 | ||
| 8 |
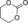
|
6 f | 1 | 79 | 58 | 41 |
| 9 | 2 | 97 | 80 | 75 | ||
| 10 |
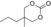
|
6 g | 1 | >99 | 98 | 94 |
| 11 |
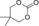
|
6 h | 1 | 80 | 73 | 57 |
[a] Conditions: 6 (0.5 mmol), H2 (30 bar), complex 1, NaOtBu (1.1 equiv with respect to 1), THF (0.7 mL), temp. (120 °C), 26 h. [b] Yield was calculated using gas chromatography, ethyl heptanoate (25 μL, 0.15 mmol) was used as an internal standard. [c] 1,3‐Propanediol (25 μL, 0.34 mmol) was used as an internal standard.
