Figure 3.
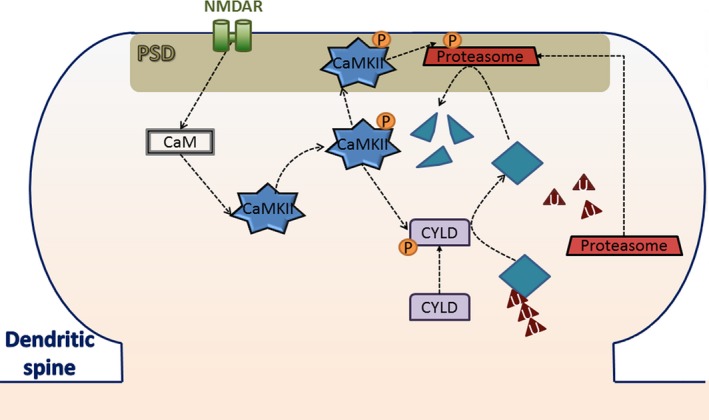
Schematic representation of how CaMKII can regulate ubiquitin proteasome system (UPS) activity and increase memory destabilization by increasing protein degradation. Calcium coming from open NMDAR binds with calmodulin creating the CaM complex that activates CaMKII. Once active, CaMKII autophosphorylates threonine 286, further increasing its activity. Active CaMKII phosphorylates cylindromatosis (CYLD), which activates this enzyme. Active CYLD removes K63‐linked polyubiquitins from proteins, targeting them for degradation. CaMKII also phosphorylates proteasome subunit Rpt6 on serine 120, increasing its activation. Active CaMKII also increases proteasome localization in the PSD. Such CaMKII/UPS pathway can help explain the behavioral phenotypes observed by Jarome et al. (2016) and Cao et al. (2008). Additionally, it is in accordance with the retrieval‐induced decrease in Shank levels, observed in Lee et al. (2008), and GluA1 (Vigil et al. 2017) levels in the post‐synaptic density.
