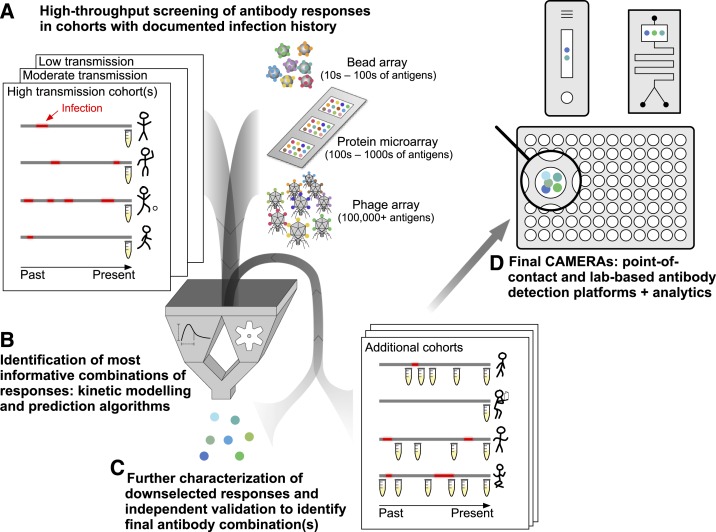
Figure 2.
Approach to designing combined antibodies to measure exposure recency assays (CAMERAs). (A) Samples from detailed cohorts, where accurate data on individuals’ prior malaria infections are available, are critical for providing a gold standard to identify informative antibody responses. Cohorts should represent the range of ages and epidemiologic settings where CAMERAs will ultimately be used. Various platforms are available for high-throughput screening of antibody responses, with tradeoffs based on cost, number of analytes that can be screened, precision, and dynamic range. (B) Down selection of the most informative combinations of responses (i.e., considered jointly) is accomplished via parametric modeling of antibody kinetics45 and/or any number of machine learning prediction algorithms. Both of these analytical approaches have advantages, and combining both may be optimal. (C) Top “hits” identified in comprehensive screens require validation in distinct individuals and cohorts. Given the smaller number of responses evaluated, it may be feasible to evaluate much larger numbers of samples including longitudinal sampling from individuals over time. (D) Final CAMERAs can be designed as point-of-contact (e.g., based on lateral flow or microfluidics) or laboratory-based assays, depending on the use case. The analytics for deriving epidemiologically relevant metrics from antibody responses will be integral to the assay. This figure appears in color at www.ajtmh.org.