Fig. 1. VLRB N8 recognizes human Bmem and PC in blood and tonsils.
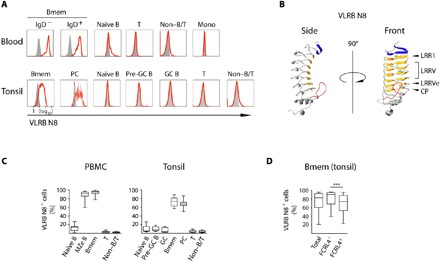
(A) Peripheral blood mononuclear cells (PBMCs) were separated into Bmem (CD19+/IgD−/CD27+) and marginal zone-equivalent (MZe) cells (CD19+/IgD+/CD27+), naïve B cells (CD19+/IgD+/CD27−), non–B/T cells (CD19−/CD3−), and T cells (CD19−/CD3+). A representative of 12 examined PBMC samples is shown. Human tonsillar lymphocytes were separated into the following subpopulations: Bmem (CD19+/IgD−/CD38−), PC (CD19+/IgD−/CD38++), naïve B cells (CD19+/IgD+/CD38−), pre-GC B cells (CD19+/IgD+/CD38+), GC B cells (CD19+/IgD−/CD38+), non–B/T cells (CD19−/CD3−), and T cells (CD19−/CD3+). A representative example of 14 analyzed tonsil samples is shown. VLRB N8 reactivity by flow cytometric analysis is indicated by solid red lines, and VLR4 reactivity (specific for the BclA antigen of the exosporium of Bacillus anthracis) as a negative control is shown as solid gray histogram. (B) Ribbon model of a monomeric antigen-binding unit of VLRB N8. Parallel β sheets lining the inner concave surface encoded by the N-terminal capping LRR are shown in blue, and sequences encoded by the LRR1, variable LRRV1-4, LRRVe and connecting peptide (CP) units are depicted in orange. A variable loop protruding from the capping C-terminal LRR is shown in red. The model was generated using the IntFOLD modeling platform (49). (C) Frequencies of VLRB N8–reactive cells for each analyzed cell population in healthy blood and tonsil samples. (D) Frequencies of VLRB N8–reactive cells between FCRL4+ and FCRL4− Bmem from 14 additional tonsil specimens. Statistical significance was assessed using a Wilcoxon signed-rank test, ***P < 0.001; n = 14.
