Fig. 1. Ponatinib directly inhibits MEKK3 kinase activity.
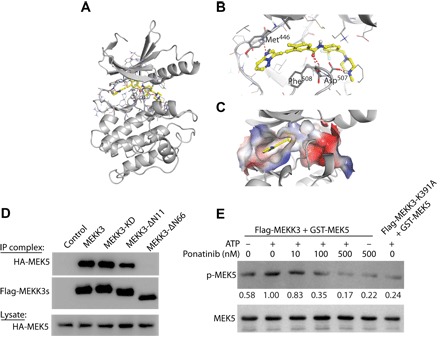
(A to C) Computer modeling demonstrating that ponatinib can bind with the MEKK3 kinase domain in the ATP-binding pocket with hydrogen-bonds and π-π interaction (color) as demonstrated by a ribbon plot with full-length proteins (A) and the ribbon plot (B) and surface plot (C) of the binding pocket. (D) Immunoprecipitation (IP) demonstrating robust MEKK3 and MEK5 interactions. Deletion of 66 amino acids at the N terminus (MEKK3-ΔN66) abolishes the interaction. Deletion of 11 amino acids at the N terminus (MEKK3-ΔN11) or kinase-dead MEKK3 (MEKK3-KD) do not affect MEKK3-MEK5 interaction. (E) In vitro kinase assays showing changes in MEK5 phosphorylation following treatment with ponatinib. MEKK3-K391A, a kinase-dead mutant, was included as a negative control. The quantification of p-MEK5/MEK5 density ratios was shown between the blots. Results are representative of three independent experiments.
