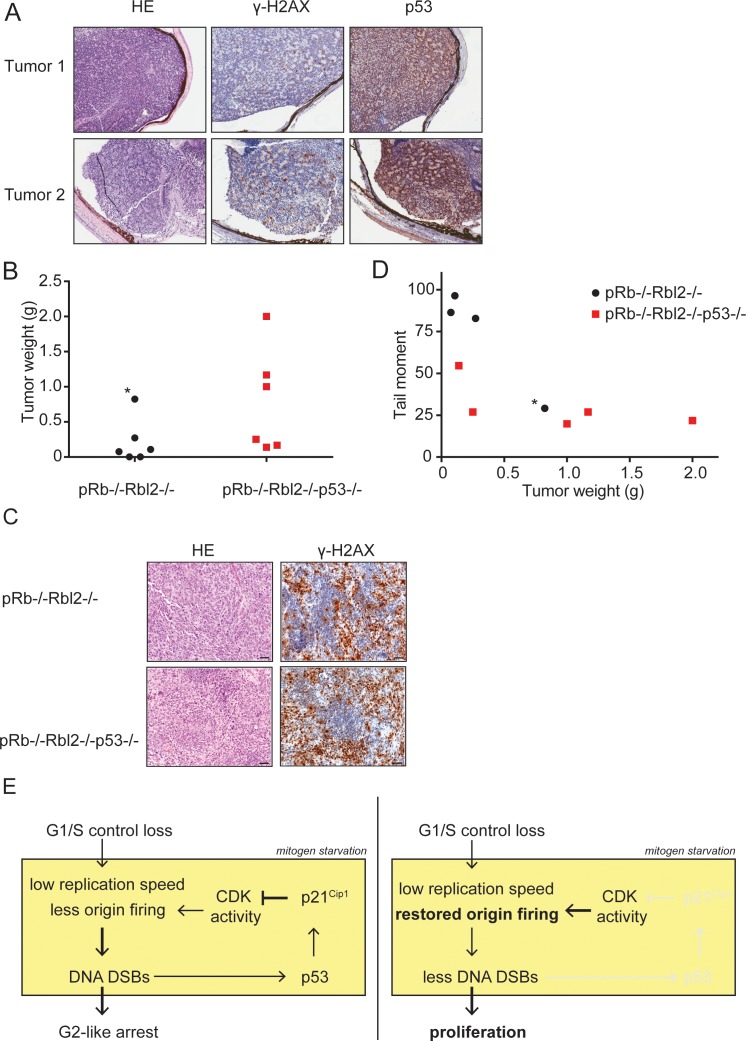
Figure 7. p53 inactivation promotes tumor growth in vivo.
(A) Examples of HE and immunohistochemical stainings for γ-H2AX and p53 of two retinoblastomas from Rb-/-Rbl2-/- chimeric mice. (B) Tumor weight of Rb-/-Rbl2-/- (black) and Rb-/-Rbl2-/-p53-/- (red) teratomas 20 days after injection of ESCs in nude mice. Black dot marked with an asterisk (*) indicates the only tumor with high levels of infiltrating neutrophils. (C) Tail moments of Rb-/-Rbl2-/- (black) and Rb-/-Rbl2-/-p53-/- (red) teratomas plotted against the tumor weight. Spearman’s correlation coefficient between tail moment and tumor weight is −0.78. Black dot marked with an asterisk (*) indicates the only tumor with high levels of infiltrating neutrophils. One Rb-/-Rbl2-/-p53-/- (red) teratoma could not be analyzed for DNA DSBs due to the small tissue size. For each teratoma, more than 50 cells were analyzed using the CASP software. (D) Examples of HE and immunohistochemical staining for γ-H2AX of Rb-/-Rbl2-/- and Rb-/-Rbl2-/-p53-/- teratomas. Scale bar is 50 µm. (E) Schematic model for how p53 inactivation reduces DNA DSBs in mitogen-deprived cells lacking the G1/S checkpoint. Cells that lost the G1/S phase checkpoint suffer from replication stress leading to DNA DSBs. Activation of p53 and p21Cip1 inhibits CDK activity and thereby inhibits the firing of new origins leading to more DNA damage and establishment of the G2-like arrest (left panel). Inactivation of p53 and therefore its downstream protein p21Cip1 increases CDK activity and allows origins to fire. Restored levels of origin firing rescues stalled forks, causing less DNA DSB formation and enabling proliferation (right panel).