Abstract
Background:
Programmed cell death ligand 1 (PD-L1) overexpression has been reported to be associated with poor prognosis in several human cancers. However, studies on the prognostic value of PD-L1 expression in ovarian carcinoma (OC) remain controversial. This meta-analysis aimed to evaluate comprehensively the prognostic value of PD-L1 in OC.
Methods:
Electronic databases, including PubMed, EMBASE, and the Cochrane Library, were searched up until March 28, 2018. Hazard ratio (HR), along with 95% confidence interval (CI), was used to analyze the included outcomes.
Results:
A total of 10 studies with 1179 OC patients were included in this meta-analysis. There was no significant correlation between PD-L1 expression and overall survival (OS) (HR 1.23, 95% CI 0.85–1.79) and progression-free survival (PFS) (HR 0.88, 95% CI 0.52–1.47) of OC patients. However, the subgroup analysis suggested that positive PD-L1 expression was significantly associated with poor OS (HR 1.66, 95% CI 1.08–2.55) and PFS (HR 2.17, 95% CI 1.31–3.61) among OC patients from Asian countries. Increased PD-L1 expression was also a favorable factor for OS (HR 0.73, 95% CI 0.53–0.99) and PFS (HR 0.58, 95% CI 0.45–0.75) in OC patients from non-Asian regions. No evidence of publication bias was detected by the Egger linear regression test and Begg funnel plot. Sensitivity analyses suggested that the results of this meta-analysis were robust.
Conclusions:
The results indicated that PD-L1 expression may be a negative predictor for prognosis of OC patients from Asian countries, and a good predictor for favorable prognosis of OC patients from non-Asian countries. PD-L1 expression has potential to be a prognostic biomarker to guide clinicians for the selection of individuals who may get clinical benefit from anti-PD-1/PD-L1 immunotherapy. Prospective clinical studies are needed to support these findings.
Keywords: meta-analysis, ovarian carcinoma, prognostic significance, programmed cell death ligand 1, survival
1. Introduction
Ovarian carcinoma (OC) is 1 of the 3 malignant tumors in gynecology and has the highest mortality rate among all gynecologic malignancies.[1] Statistically, an estimated 238,700 new OC cases occurred, and 151,900 patients died of OC in 2012.[2] Most of OC patients were diagnosed at an advanced stage due to the lack of specific symptoms and ways for early screening, and died of tumor recurrence and platinum resistance.[3] The 5-year survival rate is only 20% to 30% in advanced patients.[4] Over the past few decades, despite advances in cytoreductive radical surgery and all kinds of chemotherapy, only marginal improvement has been seen in the overall survival (OS) of patients with OC.[5] Therefore, it is urgently needed that precise and feasible prognostic factors are identified and validated to best guide personalized treatment and improve patient outcomes.
Programmed cell death ligand 1 (PD-L1; B7-H1; CD274) is a surface glycoprotein belonging to the B7/CD28 costimulatory factor superfamily,[6] and constitutively expressed on specific tumor and immune cells.[7] Recently, PD-L1 was considered to be up-regulated in various tumors and low expression or nonexpression in normal tissues, and was demonstrated to be involved in the immune escape mechanism of cancer cells.[8,9] Interactions between PD-L1 and its receptor, programmed cell death 1 (PD-1), can inhibit T-cell activation and cytokine production, and promote the apoptosis or exhaustion of T cells, resulting in tumor growth.[7,8,10] Blockade of the PD-1/PD-L1 signaling pathway with targeted monoclonal antibodies had become a promising therapeutic method in cancers, demonstrating encouraging antitumor activity and increasing survival rates in multiple tumor types.[10] Similarly, anti-PD-1/PD-L1 antibodies have been considered to play a significant role in adjuvant treatment of OC.[11,12] Ongoing researches are performed to identify if PD-L1 detected via immunohistochemistry (IHC) in tumor tissues could predict the curative effect of anti-PD-1/PD-L1 therapy. Increasing studies have shown that PD-L1 overexpression was associated with poor prognosis and resistance to immune therapies in several human cancers.[13–16] The prognosis significance of PD-L1 in OC patients also has been widely studied and remains controversial.[17–26] To the authors’ knowledge, no systematic review on this topic has been published so far, so the meta-analysis was conducted to evaluate comprehensively prognostic value of PD-L1 expression in OC patients.
2. Materials and methods
2.1. Ethics approval
Ethics approval was not necessary for this meta-analysis because participants have not been affected directly.
2.2. Literature search
Two authors (LJH and FC) independently performed a comprehensive literature retrieval using the PubMed, EMBASE, and Cochrane Library databases. The final search was conducted on March 28, 2018. Discrepancies were resolved by discussion with the third appraiser. The following keywords were used for literature search: “PD-L1” or “PDL1” or “B7-H1” or “B7 homolog 1” or “CD274” or “Programmed Death Ligand 1”; and “ovarian/ovary carcinoma” or “ovarian/ovary neoplasm (s)” or “ovarian/ovary cancer (s)” or “ovarian/ovary tumor (s)”. The references of the retrieved relevant articles were also screened.
2.3. Eligibility criteria
Literature inclusion criteria included the following: studies were focused on OC; all patients were histologically confirmed to have OC; PD-L1 expression was detected via IHC staining on tumor cells, and/or tumor-infiltrating lymphocytes (TILs) and/or immune cells in primary cancer tissues; PD-L1 protein expression; the studies provided an association between PD-L1 expression and prognosis; and articles were published with a full paper in English. Studies that did not meet the inclusion criteria and reported in reviews, conference abstracts, or letters were excluded. Only the study with the newest and most related information was included when duplicate publications from the same center were identified. Disagreements were resolved by discussion with a third reviewer.
2.4. Data extraction
Two authors (LJH and FC) independently extracted the following information from each included study: name of the first author, publication year, country of origin, tumor type, number of patients, Federation of Gynecology and Obstetrics (FIGO) stage, IHC staining cells and pattern, cut-off value for positive PD-L1 expression, antibody, positive expression rate of PD-L1, survival outcome and data, and quality assessment score. Discrepancies were resolved by discussion with a third reviewer. The primary outcome measures were OS and/or progression-free survival (PFS). In cases where hazard ratios (HRs) and confidence intervals (CIs) were not reported, methods described by Tierney et al[27] were used to estimate HRs. When both univariate and multivariate analyses were reported, HRs and CIs were preferentially extracted from multivariate analysis.
2.5. Assessment of study quality
Two authors (LJH and FC) independently assessed the quality of included studies using the Newcastle–Ottawa Quality Assessment Scale (NOS). Disagreements in scoring were resolved by discussion with a third reviewer. The NOS evaluates the following 3 parameters: selection (0–4 points), comparability (0–2 points), and outcome (0–3 points). The highest NOS score is 9 points, and studies scoring greater than 5 were classified as high-quality.
2.6. Statistical methods
Pooled HRs with CIs were used to evaluate the association between PD-L1 expression, and OS and PFS. Subgroup analysis was conducted based on tumor type, region, IHC staining cells, positive expression rate of PD-L1, and sample size. Heterogeneity among studies was assessed using the chi-square test and I2 metric. Random-effects model was implemented when significant heterogeneity (I2 > 50% or P < .1) was detected. Further analysis was carried out to identify the origin of heterogeneity. Sensitivity analysis was performed to evaluate the influence of single study on pooled results and to find the causes of heterogeneity. Potential publication bias was assessed using the Egger linear regression test and Begg funnel plot. This meta-analysis was performed with STATA 12.0 (StataCorp LP, College Station, TX). P < .05 were considered as statistically significant.
3. Results
3.1. Search results
A total of 385 articles were found using the above search strategy. After removing duplicate studies, 282 studies remained. After screening the titles and abstracts, 268 articles were excluded on the basis that they were not original papers (eg, reviews, letters, case reports, or conference abstracts), not ovarian carcinoma-related articles, not English language studies, not human studies, and not relevant. After reading 14 potentially eligible studies in detail, 10 articles satisfied the inclusion criteria finally. Details of the screening process are described in Fig. 1.
Figure 1.

Flow diagram of study selection.
3.2. Study characteristics
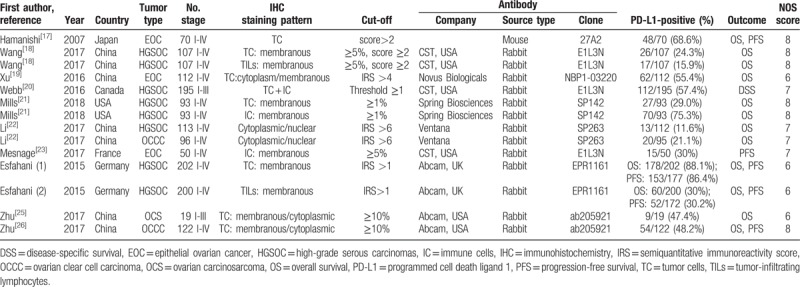
The characteristics of included studies are shown in Table 1. The studies were generally high-quality, with all NOS scores more than 5. Of note, the 2 articles by Wang et al and Esfahani et al provided 2 independent comparisons on the basis that tumor cells and TILs PD-L1 staining were scored and reported separately; the article by Mills et al showed 2 independent comparisons based on tumor cells and tumor-associated immune cells (ICs) PD-L1 staining separately; the article by Li et al provided 2 independent comparisons on the basis that PD-L1 staining in ovarian clear cell carcinoma (OCCC) and ovarian high-grade serous carcinoma (HGSC) was scored and reported separately. Thus, in total, 14 comparisons (from 10 articles) comprising 1179 patients were included in the pooled analysis. The studies were conducted in 8 countries (China, Japan, Germany, France, Canada, and the United States) and published from 2006 to 2018. All PD-L1 expression levels were detected by IHC staining. Each study had a specific cut-off value to decide positive and negative PD-L1 expression. In this review, patients were considered as positive PD-L1 expression based on the distinct cut-off criteria reported in each paper. OS and PFS were used as the endpoint in 12 and 5 of the 14 comparisons, respectively. Disease-specific survival (DSS) was evaluated in 1 study.[20] Three comparisons included patients diagnosed with epithelial ovarian carcinoma (EOC), whereas remaining comparisons included patients with the subtypes of EOC: 8 comparisons included patients with HGSOC, 2 comparisons included patients with OCCC, 1 comparison included patients with ovarian carcinosarcoma (OCS). The HR with 95% CI was calculated according to the methods provided by Tierney et al in the study by Zhu et al.[25] The studies by Hamanishi et al and by Xu et al provided the relative risk (RR) with 95% CI from Cox multifactor regression analysis using Cox hazard model, which were pooled with HRs together in this meta-analysis.
Table 1.
Characteristics of include studies for meta-analysis.
3.3. Association between PD-L1 expression and OS of OC patients
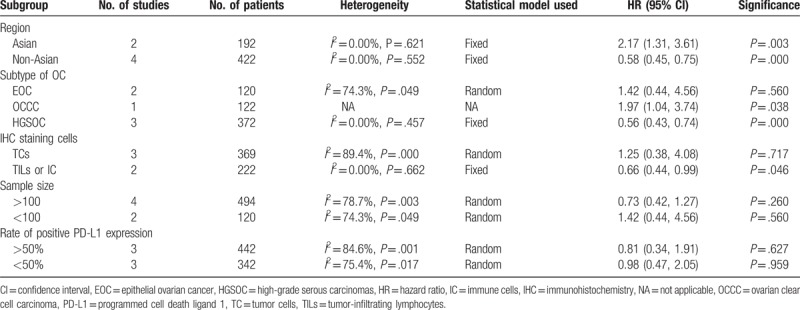
This study evaluated the association between PD-L1 expression and OS from 8 studies (involving 12 comparisons). The data were pooled with random-effects model due to significant heterogeneity (I2 = 65.9%, P = .001) among studies. The results indicated that positive PD-L1 expression was not significantly associated with OS of OC patients (HR 1.23, 95% CI 0.85–1.79) (Fig. 2). Subgroup analysis was adopted on region. The results showed that positive PD-L1 expression was associated with shorter OS of OC patients from Asian countries (HR 1.66, 95% CI 1.08–2.55), but was associated with longer OS in patients from non-Asian countries. Subgroup analysis based on the subtypes of OC showed significant association between PD-L1 expression and shorter OS in patients diagnosed with EOC and OCCC, whereas no significant correlation was observed for patients with HGSOC. Subgroup analyses were also performed by IHC staining cells, positive expression rate of PD-L1, and sample size. However, the results demonstrated that there was no significant association between PD-L1 expression and OS of OC patients in all the subgroups. The results of subgroup analyses about OS are shown in Table 2.
Figure 2.
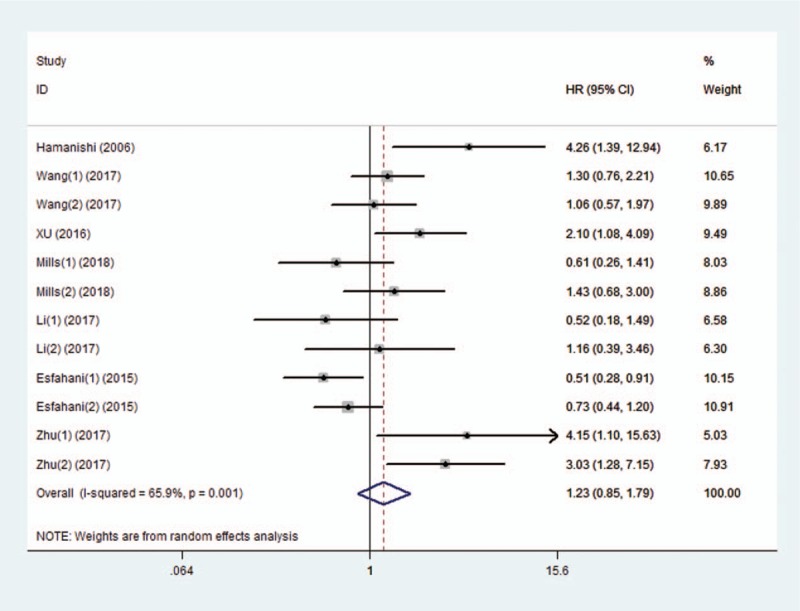
Forest plot of studies evaluating the association between programmed cell death ligand 1 (PD-L1) expression and overall survival (OS) in patients with ovarian carcinoma (OC).
Table 2.
Subgroup analysis on the outcome of overall survival.

3.4. Association between PD-L1 expression and PFS of OC patients
Six comparisons from 5 studies provided the data on PFS. A random-effects model was used to pool the data due to significant heterogeneity (I2 = 78.2%, P = .000). The results showed that there was no correlation between positive PD-L1 expression and PFS in OC patients (HR 0.88, 95% CI 0.52–1.47) (Fig. 3). In the subgroup analysis based on region, positive PD-L1 expression was significantly related to poorer PFS of OC patients from Asian countries (HR 2.17, 95% CI 1.31–3.61, I2 = 0.0%), but longer PFS in patients from non-Asian (HR 0.58, 95% CI 0.45–0.75, I2 = 0.0%). Moreover, the heterogeneity was greatly decreased. Obvious association was observed between positive PD-L1 expression and longer PFS in patients diagnosed with HGSOC (HR 0.56, 95% CI 0.43–0.74, I2 = 0.0%). Subgroup analysis by IHC staining cells was performed; the pooled results of studies detecting PD-L1 expression in nontumor cells including TILs and ICs showed that positive PD-L1 expression was significantly associated with longer PFS of OC patients (HR 0.66, 95% CI 0.44–0.99, I2 = 0.0%). The results of subgroup analyses by positive expression rate of PD-L1 and sample size showed no association between PD-L1 expression and PFS of OC patients. The results of subgroup analyses about PFS are shown in Table 3.
Figure 3.

Forest plot of studies evaluating the association between PD-L1 expression and progression-free survival (PFS) in patients with OC. OC = ovarian carcinoma, PD-L1 = programmed cell death ligand 1.
Table 3.
Subgroup analysis on the outcome of progression-free survival.
3.5. Publication bias and sensitivity analysis
Begg funnel plots and Egger regression test were used to assess the potential publication bias. As shown in the Figs. 4 and 5, the funnel plots revealed no asymmetry for OS (Begg P = .086) and PFS (Begg P = .452). The Egger tests also did not identify the publication bias in OS (Egger P = .156) and PFS (Egger P = .195). Furthermore, sensitivity analysis was conducted to assess the potential influence of each study on the pooled HRs. The results demonstrated that no study had excessive impact on the stability of the pooled results of comparisons (Fig. 6). Therefore, the results of this meta-analysis are robust.
Figure 4.

Begg funnel plot and Egger linear regression test for assessment of potential publication bias in studies investigating the correlation between PD-L1 expression and OS of OC patients. (A) Begg funnel plot; (B) Egger linear regression test. OC = ovarian carcinoma, OS = overall survival, PD-L1 = programmed cell death ligand 1.
Figure 5.
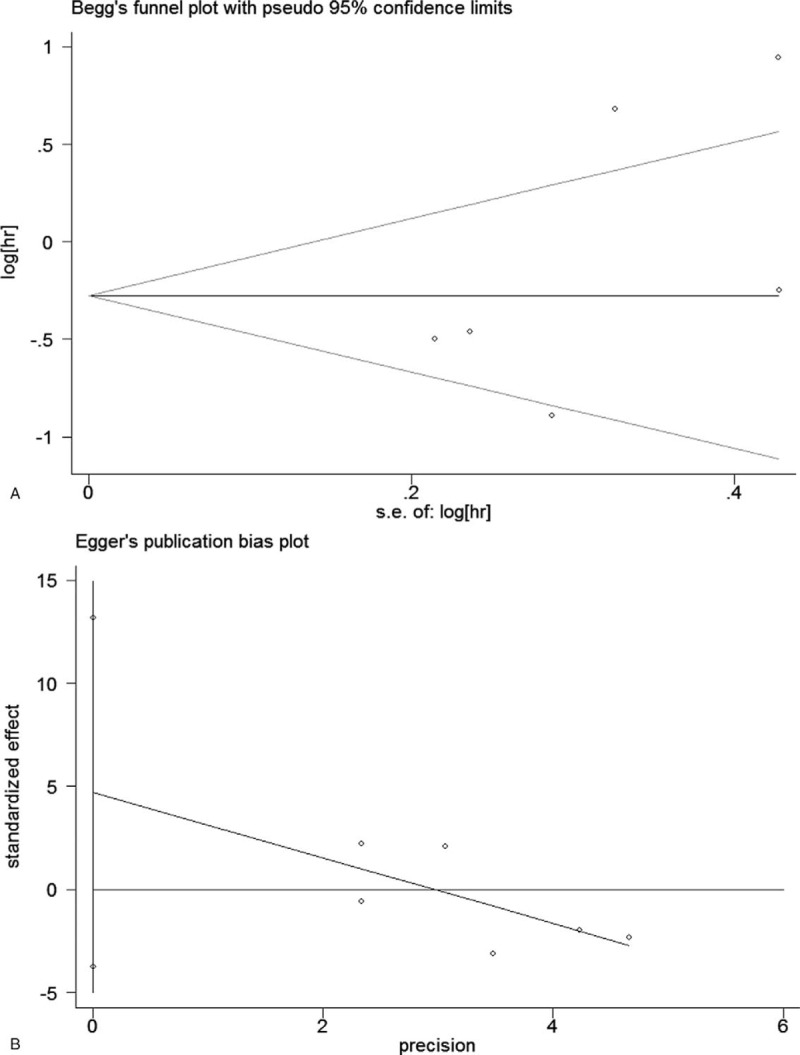
Begg funnel plot and Egger linear regression test for assessment of potential publication bias in studies investigating the correlation between PD-L1 expression and PFS of OC patients. (A) Begg funnel plot; (B) Egger linear regression test. OC = ovarian carcinoma, PD-L1 = programmed cell death ligand 1, PFS = progression-free survival.
Figure 6.

Sensitivity analysis for all eligible studies investigating the association between PD-L1 expression and OS and PFS in patients with OC. (A) Includes those studies evaluating OS; (B) includes those studies evaluating PFS. OC = ovarian carcinoma, OS = overall survival, PD-L1 = programmed cell death ligand 1, PFS = progression-free survival.
4. Discussion
To the best knowledge of the authors, the present meta-analysis is the first to investigate the relationship between PD-L1 expression and survival in OC patients. This study included 14 comparisons from 10 articles involving 1179 OC patients. The results showed that there was no association between elevated PD-L1 expression and OS or PFS in OC patients. Due to significant heterogeneity among the studies, the subgroup analysis was conducted and provided significant evidence that positive PD-L1 expression was significantly associated with poor prognosis in OC patients from Asian countries, but favorable prognosis in patients from non-Asian countries. The results of subgroup analysis by subtypes of OC showed that positive PD-L1 expression was related to poor OS in patients with EOC and OCCC, and long PFS in patients with HGSOC. When subgroup analysis based on IHC staining cells was preformed, we found that positive PD-L1 expression in TILs or ICs was associated with long PFS of OC patients. To test the stability of the results of the meta-analysis, we performed sensitive analysis on the outcomes of OS and PFS, which showed that the results of the meta-analysis were robust. However, due to the limited number of studies contained in the subgroup analyses, large prospective studies are needed to confirm the results.
This study has several advantages. First, a strict and systematic search was performed when identifying observational studies which investigated the prognostic value of PD-L1 expression in OC patients. Second, the meta-analysis was comprised of studies with similar experiment designs, which minimized methodological heterogeneity. Third, most of HRs with CIs were extracted from multivariate analysis.
There are certain limitations that need to be considered when interpreting the pooled results. First, this analysis was restricted to the studies published in English. Second, there was significant heterogeneity among the included studies. Although subgroup analyses were performed, the heterogeneity still existed, especially among the studies for OS. Third, the antibodies, assay conditions, staining pattern, tumor baseline characteristics, cut-off values used to define high or low PD-L1 expression, and scoring methods used to determine the cut-off values differed, which may be the reasons of heterogeneity. Future studies using more uniform evaluation methods will likely obtain more reliable results.
The PD-L1 overexpression has been considered to play an important role in the tumor microenvironment, with PD-1/PD-L1 pathway mainly participating in the immune escape mechanism of cancer cells and promoting growth of cancer cells.[8,28] PD-1 (CD279) is a type I transmembrane protein composed of 268 amino acids, and mainly expresses on the T cells, B cells, and nature killer (NK) cells.[29] Its ligand, PD-L1, as aforesaid, expresses on tumor cells and immune cells, including activated T and B cells, dendritic cells, and macrophages, and has been verified to be up-regulated in various tumors such as melanoma, breast cancer, nonsmall cell lung cancer, and renal cell carcinoma.[30–33] PD-L1 can specifically bind to the surface receptor molecule PD-1 of T cells, thus affecting the activation and differentiation of T cells and inhibiting the antitumor immune killing activity of T cells. The up-regulated of PD-L1 has 2 major mechanisms: the regulation of the transcription and translation levels through various intracellular signaling pathways; and the regulation of various proinflammatory factors and cytokines secreted in tumor microenvironment.[34,35] Therefore, PD-L1 was involved in the progress of cancers and may be correlated with poor prognosis of cancers. Recently, increasing meta-analyses were conducted to investigate the association between PD-L1 expression and survival in patients with tumors. Some studies showed that positive PD-L1 expression was strongly associated with poor prognosis in a number of human cancers.[36–40] However, inconsistent results have been reported in esophageal squamous cell cancer, and head and neck cancer, with no association between PD-L1 expression and survival.[41,42]
For patients with OC, the association between PD-L1 expression and prognosis was largely inconclusive. In the present analysis, 4 studies demonstrated that positive PD-L1 expression was associated with a significantly poor OS in patients with OC. Of these, the studies by Hamanishi et al and Zhu et al[26] showed significantly poor PFS in patients with OC at the same time. Seven comparisons demonstrated that there was no difference on OS between the positive PD-L1 expression and negative PD-L1 expression. Two comparisons showed that there was no correlation between positive PD-L1 expression and PFS of OC patients. Webb et al showed that positive PD-L1 expression in tumor cells and tumor-associated immune cells was correlated with DSS of HGSOC patients. In the study by Esfahani et al, the authors showed the PD-L1 expression in TILs and TC, respectively. The results showed that PD-L1 expression in TC was associated with long OS and PFS of patients with HGSOC, whereas PD-L1 expression in TILs was not associated with prognosis, which demonstrated PD-L1 expression in different cells of ovarian cancer may indicate different clinical outcomes. The difference of ethnicity, antibodies, assay conditions, staining pattern, tumor baseline characteristics, cut-off values used to define high or low PD-L1 expression, and scoring methods used to determine the cut-off values may be the reasons for irregularities of the results.
On the basis of the pooled data, we found no significant correlation between PD-L1 expression and OS or PFS of OC patients. However, subgroup analysis showed that positive PD-L1 expression was associated with poor survival in OC patients from Asian countries, but with favorable prognosis in patients from non-Asian countries. This demonstrated that PD-L1 expression as a predictor for survival in OC patients may differ in varied ethnicity. Moreover, there was no heterogeneity in the subgroup analysis by region for PFS, suggesting very reliable results. Subgroup analysis based on subtypes of OC showed that positive PD-L1 expression may correlate with poor OS in patients with EOC and OCCC, suggesting that positive PD-L1 expression may predict poor OS in these subpopulations of OC patients. These findings may help to establish the rationale for clinical studies of anti-PD-L1/PD-1 monoclonal antibodies.[43] Although anti-PD-1/PD-L1 pathway monoclonal antibodies (mAbs) have not yet been approved for the treatment of OC, growing clinical studies have shown that some of these antibodies could achieve certain efficacy. In a phase Ib clinical trial, the authors reported that Avelumab was used to treat 124 patients with refractory or recurrent OC (the dose was 10 mg/kg body weight). Consequently, 12 (9.7%) patients achieved partial remission and the disease control rate was 54%; the objective effective rate of PD-L1-positive patients was 12.3%, whereas that of PD-L1-negative patients was 5.9%.[44] Therefore, our findings may have clinical implications to guide the optimal clinical application of PD-1/PD-L1 inhibitor in OC patients from Asian countries.
5. Conclusions
In conclusion, this review is the first to report the prognostic values of PD-L1 expression in OC patients. The results showed that elevated PD-L1 expression detected via IHC in tumor tissues may be a prognostic indicator for poor survival in patients with OC from Asian countries, and for favorable prognosis from non-Asian countries. In the future, PD-L1 expression may be used to guide clinicians to select individuals who would gain durable clinical benefit from anti-PD-1/PD-L1 immunotherapy as a prognostic biomarker. Prospective clinical trials comprising optimized evaluation methods for PD-L1 expression and larger sample sizes will be necessary to confirm the regional difference on prognostic values of PD-L1 expression and to improve the outcomes of patients with OC.
Author contributions
Conceptualization: lijun huang, qizhi diao.
Data curation: lijun huang, fan chang, yang wu, xianlan wu.
Formal analysis: lijun huang, fan chang, yang wu, xianlan wu.
Funding acquisition: lijun huang, fan chang, xianlan wu.
Investigation: lijun huang, xianlan wu.
Methodology: lijun huang.
Project administration: lijun huang, fan chang.
Resources: lijun huang.
Software: lijun huang, xiaofeng deng, xianlan wu.
Supervision: lijun huang.
Validation: lijun huang, xiaofeng deng.
Visualization: lijun huang, xiaofeng deng.
Writing – original draft: lijun huang.
Writing – review & editing: lijun huang.
Footnotes
Abbreviations: 95% CI = 95% confidence interval, DSS = disease-specific survival, EOC = epithelial ovarian carcinoma, HGSC = high-grade serous carcinoma, HR = hazard ratio, IHC = immunohistochemistry, NOS = Newcastle–Ottawa Quality Assessment Scale, OC = ovarian carcinoma, OCCC = ovarian clear cell carcinoma, OCS = ovarian carcinosarcoma, OS = overall survival, PD-L1 = programmed cell death ligand 1, TILs = tumor-infiltrating lymphocytes.
The authors report no conflicts of interest in this work.
References
- [1].Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin 2017;67:7–30. [DOI] [PubMed] [Google Scholar]
- [2].Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87–108. [DOI] [PubMed] [Google Scholar]
- [3].Jayson GC, Kohn EC, Kitchener HC, et al. Ovarian cancer. Lancet 2014;384:1376–88. [DOI] [PubMed] [Google Scholar]
- [4].Howlader N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review, 1975-2014, 2017. https://seer.cancer.gov/csr/1975_2014/, based on November 2016 SEER data submission. [Google Scholar]
- [5].Nezhat FR, Apostol R, Nezhat C, et al. New insights in the pathophysiology of ovarian cancer and implications for screening and prevention. Am J Obstet Gynecol 2015;213:262–7. [DOI] [PubMed] [Google Scholar]
- [6].Reiss KA, Forde PM, Brahmer JR. Harnessing the power of the immune system via blockade of PD-1 and PD-L1: a promising new anticancer strategy. Immunotherapy 2014;6:459–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Ceeraz S, Nowak EC, Noelle RJ. B7 family checkpoint regulators in immune regulation and disease. Trends Immunol 2013;34:556–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 2012;12:252–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Taube JM, Anders RA, Young GD, et al. Colocalization of inflammatory response with B7-h1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci Transl Med 2012;4:127ra37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Zou W, Wolchok JD, Chen L. PD-L1 (B7-H1) and PD-1 pathway blockade for cancer therapy: mechanisms, response biomarkers and combinations. Sci Transl Med 2016;8:328rv4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Brahmer JR, Tykodi SS, Chow LQ, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med 2012;366:2455–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Hamanishi J, Mandai M, Ikada T, et al. Safety and antitumor activity of anti-PD-1 antibody, nivolumab, in patients with platinum-resistant ovarian cancer. J Clin Oncol 2015;33:4015–22. [DOI] [PubMed] [Google Scholar]
- [13].Chen L, Deng H, Lu M, et al. B7-H1 expression associates with tumor invasion and predicts patient's survival in human esophageal cancer. Int J Clin Exp Pathol 2014;7:6015–23. [PMC free article] [PubMed] [Google Scholar]
- [14].Muenst S, Schaerli AR, Gao F, et al. Expression of programmed death ligand 1 (PD-L1) is associated with poor prognosis in human breast cancer. Breast Cancer Res Treat 2014;146:15–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Frigola X, Inman BA, Lohse CM, et al. Identification of a soluble form of B7-H1 that retains immunosuppressive activity and is associated with aggressive renal cell carcinoma. Clin Cancer Res 2011;17:1915–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Zhou ZJ, Zhan P, Song Y. PD-L1 over-expression and survival in patients with non-small cell lung cancer: a meta-analysis. Transl Lung Cancer Res 2015;4:203–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Hamanishi J, Mandai M, Iwasaki M, et al. Programmed cell death 1 ligand 1 and tumor-infiltrating CD8+ T lymphocytes are prognostic factors of human ovarian cancer. Proc Natl Acad Sci USA 2007;104:3360–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Wang Q, Lou W, Di W, et al. Prognostic value of tumor PD-L1 expression combined with CD8(+) tumor infiltrating lymphocytes in high grade serous ovarian cancer. Int Immunopharmacol 2017;52:7–14. [DOI] [PubMed] [Google Scholar]
- [19].Xu M, Zhang B, Zhang M, et al. Clinical relevance of expression of B7-H1 and B7-H4 in ovarian cancer. Oncol Lett 2016;11:2815–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Webb JR, Milne K, Kroeger DR, et al. PD-L1 expression is associated with tumor-infiltrating T cells and favorable prognosis in high-grade serous ovarian cancer. Gynecol Oncol 2016;141:293–302. [DOI] [PubMed] [Google Scholar]
- [21].Mills AM, Peres LC, Meiss A, et al. Targetable immune regulatory molecule expression in high-grade serous ovarian carcinomas in african american women: a study of PD-L1 and IDO in 112 cases from the African American Cancer Epidemiology Study (AACES). Int J Gynecol Pathol 2018;doi: 10.1097/PGP.0000000000000494 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Li M, Li H, Fei L, et al. Characterization of ovarian clear cell carcinoma using target drug-based molecular biomarkers: implications for personalized cancer therapy. J Ovarian Res 2017;10:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Mesnage SJL, Auguste A, Genestie C, et al. Neoadjuvant chemotherapy (NACT) increases immune infiltration and programmed death-ligand 1 (PD-L1) expression in epithelial ovarian cancer (EOC). Ann Oncol 2017;28:651–7. [DOI] [PubMed] [Google Scholar]
- [24].Darb-Esfahani S, Kunze CA, Kulbe H, et al. Prognostic impact of programmed cell death-1 (PD-1) and PD-ligand 1 (PD-L1) expression in cancer cells and tumor-infiltrating lymphocytes in ovarian high grade serous carcinoma. Oncotarget 2016;7:1486–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Zhu J, Hao W, Ju X, et al. Clinical significance of programmed death ligand-1 and intra-tumoral CD8+ T lymphocytes in ovarian carcinosarcoma. PloS One 2017;12:e0170879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Zhu J, Wen H, Bi R, et al. Prognostic value of programmed death-ligand 1 (PD-L1) expression in ovarian clear cell carcinoma. J Gynecol Oncol 2017;28:e77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Tierney JF, Stewart LA, Ghersi D, et al. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 2007;8:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Kleffel S, Posch C, Barthel SR, et al. Melanoma cell-intrinsic PD-1 receptor functions promote tumor growth. Cell 2015;162:1242–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Riella LV, Paterson AM, Sharpe AH, et al. Role of the PD-1 pathway in the immune response. Am J Transplant 2012;12:2575–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Posabella A, Schaerli A, Muenst S, et al. The expression of programmed death ligand 1 (PD-L1) is associated with poor prognosis in human breast cancer. Breast Cancer Res Treat 2014;146:15–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Yang CY, Lin MW, Chang YL, et al. Programmed cell death-ligand 1 expression in surgically resected stage I pulmonary adenocarcinoma and its correlation with driver mutations and clinical outcomes. Eur J Cancer 2014;50:1361–9. [DOI] [PubMed] [Google Scholar]
- [32].Choueiri TK, Fay AP, Gray KP, et al. PD-L1 expression in non clear-cell renal cell carcinoma. Ann Oncol 2014;25:2178–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Madore J, Vilain RE, Menzies AM, et al. PD-L1 expression in melanoma shows marked heterogeneity within and between patients: implications for anti-PD-1/PD-L1 clinical trials. Pigment Cell Melanoma Res 2015;28:245–53. [DOI] [PubMed] [Google Scholar]
- [34].Alsaab HO, Sau S, Alzhrani R, et al. PD-1 and PD-L1 checkpoint signaling inhibition for cancer immunotherapy: mechanism, combinations, and clinical outcome. Front Pharmacol 2017;8:561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Guan J, Lim KS, Mekhail T, et al. Programmed death ligand-1 (PD-L1) expression in the programmed death receptor-1 (PD-1)/PD-L1 blockade: a key player against various cancers. Arch Pathol Lab Med 2017;141:851–61. [DOI] [PubMed] [Google Scholar]
- [36].Wang Z, Peng S, Xie H, et al. Prognostic and clinicopathological significance of PD-L1 in patients with renal cell carcinoma: a meta-analysis based on 1863 individuals. Clin Exp Med 2018;18:165–75. [DOI] [PubMed] [Google Scholar]
- [37].Gao HL, Liu L, Qi ZH, et al. The clinicopathological and prognostic significance of PD-L1 expression in pancreatic cancer: A meta-analysis. Hepatobiliary Pancreat Dis Int 2018;17:95–100. [DOI] [PubMed] [Google Scholar]
- [38].Aghajani M, Graham S, McCafferty C, et al. Clinicopathologic and prognostic significance of programmed cell death ligand 1 expression in patients with non-medullary thyroid cancer: a systematic review and meta-analysis. Thyroid 2018;28:349–61. [DOI] [PubMed] [Google Scholar]
- [39].Ma G, Deng Y, Jiang H, et al. The prognostic role of programmed cell death-ligand 1 expression in non-small cell lung cancer patients: An updated meta-analysis. Clin Chem Acta 2018;482:101–7. [DOI] [PubMed] [Google Scholar]
- [40].Li X, Li M, Lian Z, et al. Prognostic role of programmed death ligand-1 expression in breast cancer: a systematic review and meta-analysis. Target Oncol 2016;11:753–61. [DOI] [PubMed] [Google Scholar]
- [41].Qu HX, Zhao LP, Zhan SH, et al. Clinicopathological and prognostic significance of programmed cell death ligand 1 (PD-L1) expression in patients with esophageal squamous cell carcinoma: a meta-analysis. J Thorac Dis 2016;8:3197–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Li J, Wang P, Xu Y. Prognostic value of programmed cell death ligand 1 expression in patients with head and neck cancer: a systematic review and meta-analysis. Plos One 2017;12:e0179536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Gaillard SL, Secord AA, Monk B. The role of immune checkpoint inhibition in the treatment of ovarian cancer. Gynecol Oncol Res Pract 2016;3:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Disis MLPM, Pant S, Hamilton EP, et al. Avelumab(MSB0010718C; antiPD-L1) in patients with recurrent/refractory ovarian cancer from the JAVELIN Solid Tumor phase Ib trial: safety and clinical activity. J Clin Oncol 2016;34:5533. [Google Scholar]


