Abstract
Background:
Postoperative delirium is a prevalent and disabling mental disorder in patients undergoing on-pump cardiac surgery. There is some evidence that the use of pharmacological interventions may reduce the risk of developing of postoperative delirium. Therefore, the aim of this meta-analysis was to determine the effect of pharmacologic agents for the prevention postoperative delirium after cardiac surgery.
Methods:
Randomized controlled trials (RCTs) were identified through a systematic literature search of electronic databases and article references up to October 2016. End points included incidence of postoperative delirium, severity of postoperative delirium, cognitive disturbances of postoperative delirium, duration of postoperative delirium, length of stay in intensive care unit (ICU) and hospital, and short-term mortality.
Results:
A total of 14 RCTs with an aggregate of 14,139 patients were included. The results of the present meta-analysis show that pharmacologic agents significantly decrease postoperative delirium [relative risk (RR), 0.83; 95% confidence interval (95% CI), 0.75–0.91, P < .00001] and duration of postoperative delirium (RR = −0.37, 95% CI = −0.47 to −0.27, P < .00001) after on-pump cardiac surgery. In addition, subgroup analysis shows that dexamethasone and dexamethasone were associated with a trend toward a reduction in postoperative delirium (RR, 0.45; 95% CI, 0.30–0.66, P < .0001; RR, 0.80; 95% CI, 0.68–0.93, P = .003, respectively). However, our results fail to support the assumption that pharmacologic prophylaxis is associated with a positively reduction in short-term mortality, length of ICU, or hospital stay.
Conclusion:
This meta-analysis suggests that the perioperative use of pharmacologic agents can prevent postoperative delirium development in patients undergoing cardiac surgery. However, there remain important gaps in the evidence base on a few small studies with multiple limitations. Further large-scale, high-quality RCTs are needed in this area.
Keywords: cardiac surgery, cardiopulmonary bypass, delirium, postoperative delirium, prophylaxis
1. Introduction
Postoperative delirium is a common neuropsychological syndrome encountered after cardiac surgery and has important clinical and economic implications. The incidence of postoperative delirium reported in previous studies range from 3% to 52% of patients following cardiac surgery, depending on methods of detection.[1–6] The reported incidence of delirium in elderly patients (70 years and older) undergoing cardiac surgery is as high as 54.9%.[7] Postoperative delirium is associated with increased mortality, prolonged hospital stay, costs, and increased risk of infection and stroke.[8–11]
The pathogenesis of postoperative delirium is complex and multifactorial and is for the moment far from being fully understood. Multiple risk factors have been identified, including age, previous psychiatric conditions, cerebrovascular disease, pre-existent cognitive impairment, type of surgery, perioperative blood product transfusion, administration of risperidone, postoperative atrial fibrillation, mechanical ventilation time, postoperative oxygen saturation, and renal insufficiency.[12] The most consistent predisposing risk factor of postoperative delirium is advanced age.[7,12,13]
Although no pharmacologic intervention has been approved by the Food and Drug Administration (FDA) in the treatment or prevention of delirium in any population, currently many studies have evaluated the effectiveness of pharmacologic interventions to prevent or decrease the high incidence of postoperative delirium in the cardiac surgical population.[14,15] Pharmacological interventions, including cholinesterase inhibitors, antipsychotics, and analgesics, might potentially modify the multiple neurotransmitter pathways involved in the development of delirium.[16]
Therefore, we performed a systematic review and meta-analysis of randomized controlled trials (RCTs) to assess the effectiveness of pharmacological interventions for preventing delirium after cardiac surgery with cardiopulmonary bypass.
2. Materials and methods
2.1. Search strategy and study selection
Two investigators (LP and CJ) independently searched the literatures collected in PubMed, MEDLINE, EMBASE, Science Direct, ISI Web of Knowledge, and Cochrane databases up to October 30, 2016. Search terms included “delirium,” “coronary artery bypass,” “coronary artery bypass grafting,” “heart surgery,” “cardiac surgery,” “heart valve,” “cardiopulmonary bypass,” “CPB.” We also sought additional studies by reviewing the reference lists of included articles, conference abstracts, and the bibliographies of expert advisors. The searches were limited to English publications in humans. We did not include abstracts or meeting proceedings. This search strategy was performed iteratively until no new potential citations could be found on review of the reference lists of retrieved articles.
Studies were included if they met all of the following criteria: comparative studies of pharmacologic interventions (with or without placebo) to prevent delirium after on-pump cardiac surgery in adult patients; the study design was a prospective RCT; the study reported at least 1 clinical outcome. Exclusion criteria included studies of delirium treatment, duplicate publication, ongoing/unpublished study, studies published only as an abstract or in conference proceedings, and studies without availability of detailed data.
2.2. Data extraction and quality assessment
Two review authors (YL and GG) evaluated the quality of all included studies by examining 3 items: patient selection, comparability of interventions and control groups, and assessment of outcomes. All data were extracted from article texts, tables, and figures. Two individual investigators independently extracted data on patient and study characteristics, outcomes, and study quality for each trial using a standardized protocol and reporting form. Disagreements were resolved by consensus with a third reviewer (WX). The following study parameters were extracted: primary author, publication year, country of origin, intervention, diagnostic criteria, incidence of delirium, severity of delirium, duration of delirium, length of stay in ICU and hospital, short-term mortality, and adverse outcomes.
Methodological quality assessment was conducted by 2 authors independently. The quality of included trials was evaluated on the basis of Cochrane Risk of Bias Methods according to the following criteria as previously described[17]: random sequence generation, allocation concealment, blinding of participants and researchers, blinding of outcome assessment, incomplete outcome data, selective reporting, and other bias.
2.3. Study outcomes and statistical analysis
The primary end point of this meta-analysis was the incidence of delirium. Secondary outcomes included severity of delirium, duration of delirium, length of stay in ICU and hospital, short-term mortality, adverse outcomes, publication bias, and sensitivity analyses.
We used fixed-effects or random-effects models to produce across-study summary relative risk (RR) with 95% confidence interval (95% CI). The pooled effects were calculated using fixed-effect model with the Mantel–Haenszel method when there was no significant heterogeneity or with DerSimonian–Laird weights for the random effects model when there was significant heterogeneity. The Chi-square test was used to study heterogeneity between trials, and the I2 statistic was used to estimate the percentage of total variation across studies. I2 value greater than 50% was considered as significant heterogeneity. Publication bias was explored through visual inspection of funnel plots and assessed by applying the Egger weighted regression statistic with a P value < .05 indicating significant publication bias among the included studies. Correction for publication bias was performed using trim-and-fill methods. A P value < .05 was regarded as significant. All statistical analyses were performed using Review Manager (version 5; Cochrane Collaboration, Oxford, UK).
3. Results
3.1. Study identification and characteristics
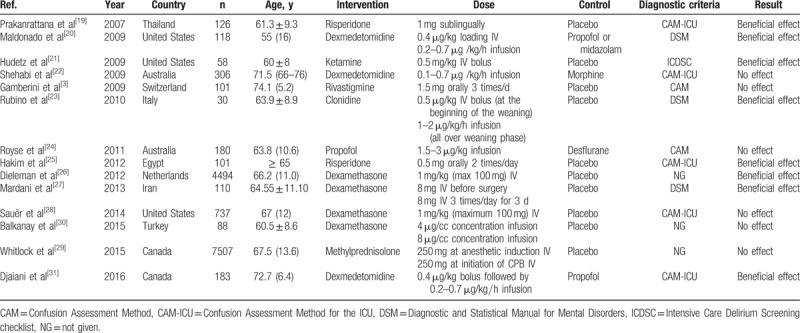
The flow diagram of literature search is presented in Fig. 1. A total of 54 studies were retrieved, and finally, 14 eligible RCTs[3,18–30] were included in this meta-analysis after abstract screening and full-text reviewing. The characteristics of the eligible RCTs in the meta-analysis are summarized in Table 1. Nine studies were placebo-controlled trials, and 4 studies evaluated a delirium prevention intervention against usual care. The years of publication of the 14 RCTs were between 2007 and 2016, and the sample sizes ranged from 30 to 7507. The surgery types included CABG, valve surgery, CABG and valve surgery, aortic surgery, and other procedures. Cardiopulmonary bypass was used in cardiac surgery in all of the trials. The average ages of the patients were all older than 60 years except 1 study by Maldonado et al.[19] The pharmacologic agents assessed in these RCTs included Risperidone (n = 2),[18,24] Dexmedetomidine (n = 4),[19,21,29,30] Ketamine (n = 1),[20] Rivastigmine (n = 1),[3] Clonidine (n = 1),[22] Propofol (n = 1),[23] Dexamethasone (n = 3),[25–27] and Methylprednisolone (n = 1).[28]
Figure 1.
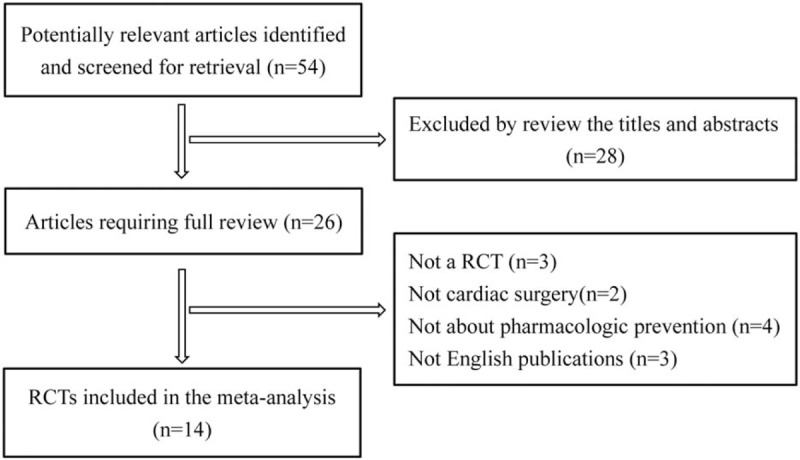
Flow diagram of studies included in the systematic review and meta-analysis.
Table 1.
Characteristics of included studies.
3.2. Incidence of postoperative delirium
Figure 2 shows the pooled results from the random effects model combining the RRs for mortality. Pooled analysis from all 14 RCTs with 14,139 patients indicated evidence of a reduction in the incidence of postoperative delirium after on-pump cardiac surgery for pharmacological interventions (RR, 0.83; 95% CI, 0.75–0.91, P = .0002), with a high level of heterogeneity among the studies (I2 = 62%, P = .001). Subgroup analysis was performed according to the pharmacologic agent prevention. The subgroup of 4 studies in which patients received dexmedetomidine was associated with significantly lower rates of postoperative delirium after cardiac surgery (RR, 0.45; 95% CI, 0.30–0.66, P < .0001). The test for heterogeneity was not significant (I2 = 38%, P = .18). Administration of dexamethasone was associated with a trend toward a reduction in postoperative delirium (RR, 0.80; 95% CI, 0.68–0.93, P = .003). The test for heterogeneity was not significant (I2 = 44%, P = .17).
Figure 2.
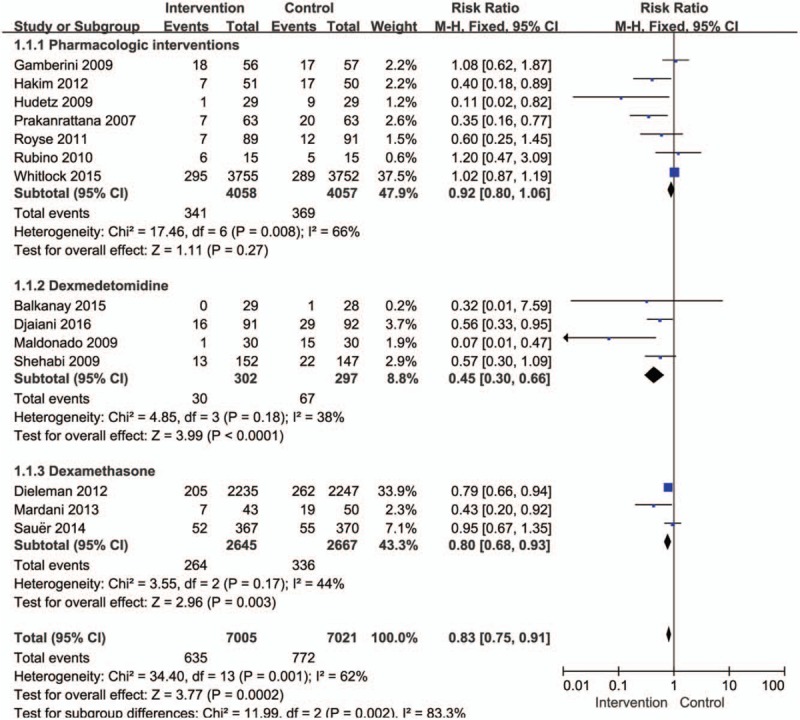
Forest plot for the meta-analysis of the incidence of postoperative delirium after cardiac surgery.
3.3. Severity of postoperative delirium
Although various instruments have been used to diagnose delirium, the diagnostic scores of some of these scales cannot be designed to assess the severity of delirium.[31] Delirium severity was reported as an outcome in only 1 RCT, which used the delirium detection score to measure the severity. Clonidine versus placebo was associated with less severe delirium (mean delirium detection score, 0.6 ± 0.7 vs 1.8 ± 0.8, respectively; P < .001).[22]
3.4. Cognitive disturbances of postoperative delirium
Disturbances in cognitive function are inherent to delirium. Three studies assessing cognitive function utilized the mini-mental status examination (MMSE) questionnaire in patients of delirium.[3,19,26] A meta-analysis using a fixed-effects model revealed that prophylactic interventions was associated with higher MMSE scores on postoperative days than placebo group (RR = 0.33, 95% CI = 0.09–0.57, P = .006, Fig. 3).
Figure 3.
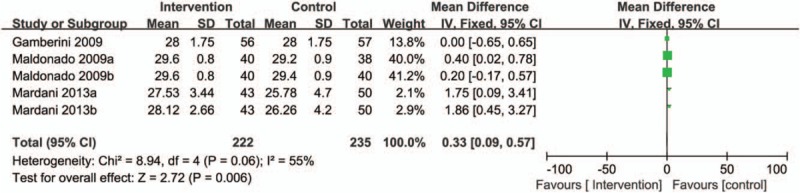
Forest plot for the meta-analysis of cognitive disturbances of postoperative delirium after cardiac surgery.
Prophylactic dexamethasone compared with placebo resulted in higher MMSE scores during the first (27.53 ± 3.44 vs 25.78 ± 4.70, respectively, P = .04) and second postoperative days (28.12 ± 2.66 vs 26.26 ± 4.20, respectively, P = .04).[26] However, an RCT by Gamberini of rivastigmine versus placebo on postoperative MMSE failed to show a difference in median scores during the first 6 postoperative days [25 (12–30) vs 24 (10–29), P = .1].[3]
3.5. Duration of postoperative delirium
The overall effect of pharmacological interventions on the duration of delirium was assessed from 5 trials. There was a significant reduction the duration of delirium in prophylactic pharmacologic agents group compared with control group (RR = −0.37, 95% CI = −0.47 to −0.27, P < .00001, I2 = 98%; Fig. 4). We did subgroup analyses based on pharmacologic agent intervention; the subgroup analysis showed dexmedetomidine significantly reduced the duration of delirium after cardiac surgery compared with control interventions (RR = −1.63, 95% CI = −1.82 to −1.43, P < .00001, I2 = 98%; Fig. 4). In contrast, there were no significant differences with regard to the duration of delirium in group receiving rivastigmine [3 (1–6) vs 2.5 (1–5) days, P = .3] or risperidone [3 days (2–4) vs 3 days (3–4), P = .664] when compared with placebo.[3,24] In addition, Sauer et al[27] conducted a randomized, placebo-controlled evaluation of high-dose dexamethasone for prevention of delirium after cardiac surgery; the median duration of delirium was similar between the dexamethasone and placebo groups [2 (1–3) vs 2 (1–2) days, 95% CI, 0.83–1.17; P = .45).
Figure 4.
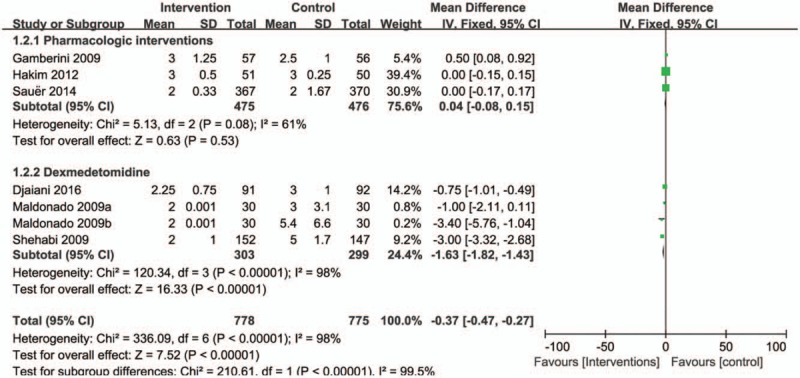
Forest plot for the meta-analysis of duration of postoperative delirium after cardiac surgery.
3.6. Length of stay in ICU and hospital
When the results of 14 RCTs that evaluated mortality as one of the outcomes were statistically aggregated, pharmacologic prevention strategies were associated with a trend toward a reduction in the length of ICU stay (RR = −0.07, 95% CI = −0.13 to −0.00, P = .04, I2 = 88%; Fig. 5). There was no subgroup meaningful difference in patients with received dexmedetomidine (RR = −0.10, 95% CI = −0.30 to −0.09, P = .29, I2 = 55%; Fig. 5) or dexamethasone (RR = 0.00, 95% CI = −0.08 to 0.08, P = .94, I2 = 88%; Fig. 5).
Figure 5.
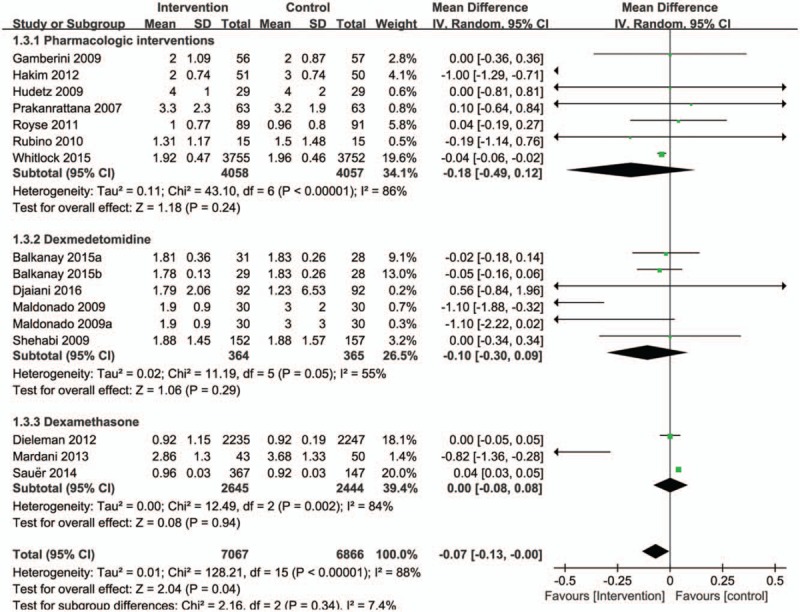
Forest plot for the meta-analysis of duration of ICU length of stay.
Furthermore, the pooled results from these studies showed no statistically significant reduction in the length of hospitalization after treatment with pharmacological agents (RR = −0.03, 95% CI = −0.07 to 0.02, P = .22, Fig. 6). There was a high level of heterogeneity between studies (I2 = 84%, P < .00001). However, subgroup analysis shows that dexamethasone was associated with a trend toward a reduction in the length of hospitalization (RR = −0.95, 95% CI = −1.19 to −0.71, P < .00001, I2 = 0%; Fig. 6).
Figure 6.
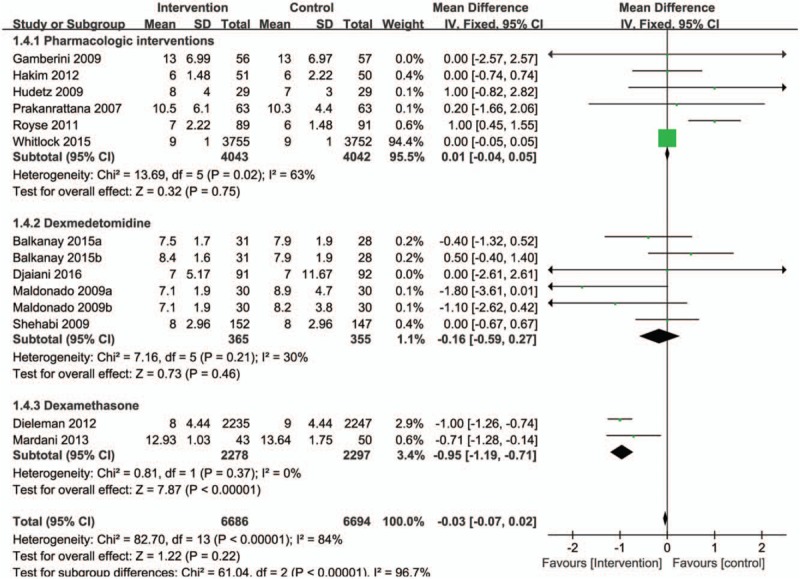
Forest plot for the meta-analysis of duration of hospital length of stay.
3.7. Short-term mortality
Seven trials reported mortality, and when these data were aggregated, the overall risk of hospital mortality was not different between pharmacologic prevention and control groups (RR = 0.92, 95% CI = 0.59–1.43, P = .61, test for heterogeneity was not significant P = .91, I2 = 0%; Fig. 7).
Figure 7.
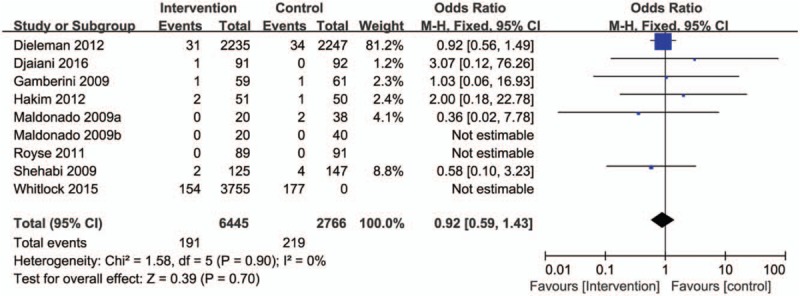
Forest plot for the meta-analysis of duration of short-term mortality.
3.8. Risk of bias in included studies
Graphical representations of the overall risk of bias in included studies are presented in Fig. 8. Risk of bias analysis showed that 1 study had a high risk of bias due to the rate of missing outcome data > 20%.[3]
Figure 8.
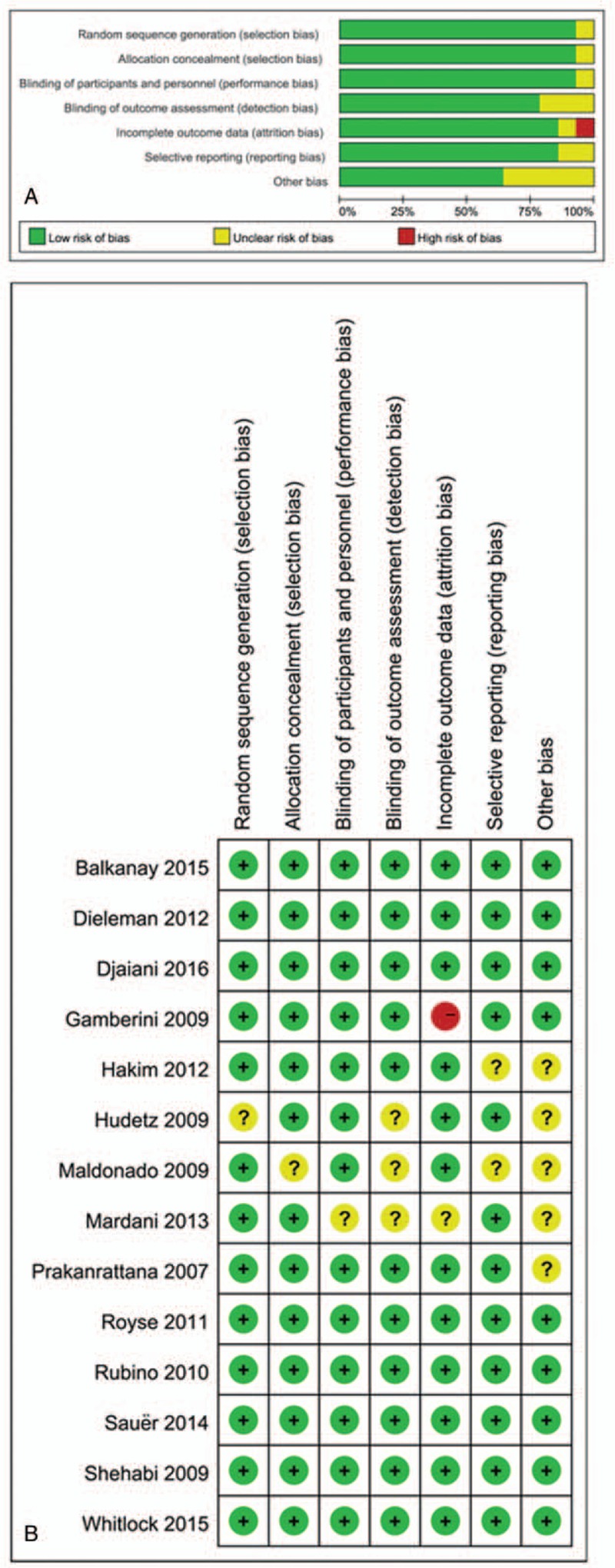
Risk of bias assessment. (A) Authors’ judgments about risk of bias graph for each included study. (B) Authors’ judgments about risk of bias summary across all included studies.
4. Discussion
Overall, this meta-analysis of RCT studies showed that pharmacologic prevention was associated with a lower risk of postoperative delirium and duration of postoperative delirium after on-pump cardiac surgery. However, our results fail to support the assumption that pharmacologic prophylaxis is associated with a positive reduction in short-term mortality and other outcomes, such as length of ICU stay and length of hospital stay. There was a huge heterogeneity of interventions among RCT trials.
Postoperative delirium is a common and dangerous complication of cardiac surgery, especially in older patients.[32] Postoperative delirium is associated with higher morbidity and mortality, prolonged hospital stay, and increased health care costs.[9,33] Currently, many risk factors for delirium have been identified, but its exact pathophysiologic mechanism remains poorly understood.[34]
A number of trials have to detect the influence of perioperative dexmedetomidine on cardiac surgery patients. Given the results of these trials, dexmedetomidine may be beneficial for preventing postoperative delirium in cardiac surgical patients. Dexmedetomidine is a highly selective, shorter-acting alpha-2 receptor agonist binding to transmembrane G protein-binding adrenoreceptors without any effect on the GABA receptor.[35] The previous meta-analysis and a current RCT study of dexmedetomidine versus placebo or other sedatives showed that dexmedetomidine-based sedation regimen resulted in reduced incidence, delayed onset, and shortened duration of postoperative delirium.[30] The results of our subgroup analysis indicated that dexamethasone was associated with a trend toward a reduction in postoperative delirium after cardiac surgery.
There are several important limitations in this study. First, clinical and statistical heterogeneity was prominently apparent in the meta-analyses; this makes interpretation and generalization across different cardiac surgery populations difficult. Second, the findings are far from robust due to most of the interventions being based on a small number of trials and small sample sizes. Third, the methodologies used for the diagnosis of delirium were different across included studies. Finally, the positive effects of the prophylactic use of pharmacologic agents may be an overestimate due to the publication bias when meta-analysis was based on previously published studies, in which positive results have more tendency to be published than negative results. Moreover, some studies suffered from methodological defects and were at a high risk of bias.
Given the prevalence and costs of postoperative delirium, there is a need for further controlled clinical trials in the treatment of this disorder. The differential efficacy and acceptability of different classes of medication, including newer agents potentially useful in this disorder, requires further study. Therefore, further large trials are required to determine the differential efficacy and acceptability of different classes of medication, including newer agents potentially useful in this disorder, and optimal methods of prophylaxis of postoperative delirium following cardiac surgery. Furthermore, future investigations should focus on pathophysiologic mechanism of postoperative delirium after cardiac surgery may help determine optimal pharmacologic targets for prophylaxis.
5. Conclusion
Although various limitations exist, our present meta-analysis clearly demonstrates the use of pharmacologic agents for the prevention of postoperative delirium development in patients undergoing cardiac surgery. The limited evidence also shows that pharmacologic prevention might also reduce the duration of postoperative delirium. However, we found no beneficial effect of pharmacologic prevention, which was associated with a positive reduction in short-term mortality, length of ICU stay, and length of hospital stay.
Author contributions
Conceptualization: Rui Tao, Xiao-Wen Wang, Yong-Mei Wang.
Data curation: Xiao-Wen Wang, Liang-Jun Pang.
Methodology: Rui Tao, Xiao-Wen Wang, Liang-Jun Pang, Jun Cheng, Guo-Qing Gao, Yu Liu, Chao Wang.
Project administration: Yong-Mei Wang.
Software: Rui Tao, Jun Cheng.
Writing – original draft: Rui Tao.
Writing – review & editing: Xiao-Wen Wang, Yong-Mei Wang.
Footnotes
Abbreviations: CABG = coronary artery bypass grafting, CPB = cardiopulmonary bypass, RCTs = randomized controlled trials.
Funding/support: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
The authors of this work have nothing to disclose and have no conflicts of interest.
References
- [1].Mariscalco G, Cottini M, Zanobini M, et al. Preoperative statin therapy is not associated with a decrease in the incidence of delirium after cardiac operations. Ann Thorac Surg 2012;93:1439–47. [DOI] [PubMed] [Google Scholar]
- [2].Bakker RC, Osse RJ, Tulen JH, et al. Preoperative and operative predictors of delirium after cardiac surgery in elderly patients. Eur J Cardiothorac Surg 2012;41:544–9. [DOI] [PubMed] [Google Scholar]
- [3].Gamberini M, Bolliger D, Lurati Buse GA, et al. Rivastigmine for the prevention of postoperative delirium in elderly patients undergoing elective cardiac surgery: a randomized controlled trial. Crit Care Med 2009;37:1762–8. [DOI] [PubMed] [Google Scholar]
- [4].Norkiene I, Ringaitiene D, Kuzminskaite V, et al. Incidence and risk factors of early delirium after cardiac surgery. BioMed Res Int 2013;2013:323491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Koster S, Hensens AG, Schuurmans MJ, et al. Prediction of delirium after cardiac surgery and the use of a risk checklist. Eur J Cardiovasc Nurs 2013;12:284–92. [DOI] [PubMed] [Google Scholar]
- [6].Rudolph JL, Jones RN, Levkoff SE, et al. Derivation and validation of a preoperative prediction rule for delirium after cardiac surgery. Circulation 2009;119:229–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Smulter N, Lingehall HC, Gustafson Y, et al. Delirium after cardiac surgery: incidence and risk factors. Interact Cardiovasc Thorac Surg 2013;17:790–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Schor JD, Levkoff SE, Lipsitz LA, et al. Risk factors for delirium in hospitalized elderly. JAMA 1992;267:827–31. [PubMed] [Google Scholar]
- [9].Milbrandt EB, Deppen S, Harrison PL, et al. Costs associated with delirium in mechanically ventilated patients. Crit Care Med 2004;32:955–62. [DOI] [PubMed] [Google Scholar]
- [10].Martin BJ, Buth KJ, Arora RC, et al. Delirium as a predictor of sepsis in post-coronary artery bypass grafting patients: a retrospective cohort study. Crit Care 2010;14:R171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Saczynski JS, Marcantonio ER, Quach L, et al. Cognitive trajectories after postoperative delirium. N Engl J Med 2012;367:30–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Gosselt AN, Slooter AJ, Boere PR, et al. Risk factors for delirium after on-pump cardiac surgery: a systematic review. Crit Care 2015;19:346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Tse L, Schwarz SK, Bowering JB, et al. Incidence of and risk factors for delirium after cardiac surgery at a quaternary care center: a retrospective cohort study. J Cardiothorac Vasc Anesth 2015;29:1472–9. [DOI] [PubMed] [Google Scholar]
- [14].Barr J, Fraser GL, Puntillo K, et al. Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the intensive care unit. Crit Care Med 2013;41:263–306. [DOI] [PubMed] [Google Scholar]
- [15].Khan BA, Gutteridge D, Campbell NL. Update on pharmacotherapy for prevention and treatment of post-operative delirium: a systematic evidence review. Curr Anesthesiol Rep 2015;5:57–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Siddiqi N, Harrison JK, Clegg A, et al. Interventions for preventing delirium in hospitalised non-ICU patients. Cochrane Database Syst Rev 2016;3:Cd005563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Higgins JP, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Prakanrattana U, Prapaitrakool S. Efficacy of risperidone for prevention of postoperative delirium in cardiac surgery. Anaesth Intensive Care 2007;35:714–9. [DOI] [PubMed] [Google Scholar]
- [19].Maldonado JR, Wysong A, van der Starre PJ, et al. Dexmedetomidine and the reduction of postoperative delirium after cardiac surgery. Psychosomatics 2009;50:206–17. [DOI] [PubMed] [Google Scholar]
- [20].Hudetz JA, Patterson KM, Iqbal Z, et al. Ketamine attenuates delirium after cardiac surgery with cardiopulmonary bypass. J Cardiothorac Vasc Anesth 2009;23:651–7. [DOI] [PubMed] [Google Scholar]
- [21].Shehabi Y, Grant P, Wolfenden H, et al. Prevalence of delirium with dexmedetomidine compared with morphine based therapy after cardiac surgery: a randomized controlled trial (DEXmedetomidine COmpared to Morphine-DEXCOM Study). Anesthesiology 2009;111:1075–84. [DOI] [PubMed] [Google Scholar]
- [22].Rubino AS, Onorati F, Caroleo S, et al. Impact of clonidine administration on delirium and related respiratory weaning after surgical correction of acute type-A aortic dissection: results of a pilot study. Interact Cardiovasc Thorac Surg 2010;10:58–62. [DOI] [PubMed] [Google Scholar]
- [23].Royse CF, Andrews DT, Newman SN, et al. The influence of propofol or desflurane on postoperative cognitive dysfunction in patients undergoing coronary artery bypass surgery. Anaesthesia 2011;66:455–64. [DOI] [PubMed] [Google Scholar]
- [24].Hakim SM, Othman AI, Naoum DO. Early treatment with risperidone for subsyndromal delirium after on-pump cardiac surgery in the elderly: a randomized trial. Anesthesiology 2012;116:987–97. [DOI] [PubMed] [Google Scholar]
- [25].Dieleman JM, Nierich AP, Rosseel PM, et al. Intraoperative high-dose dexamethasone for cardiac surgery: a randomized controlled trial. JAMA 2012;308:1761–7. [DOI] [PubMed] [Google Scholar]
- [26].Mardani D, Bigdelian H. Prophylaxis of dexamethasone protects patients from further post-operative delirium after cardiac surgery: a randomized trial. J Res Med Sci 2013;18:137–43. [PMC free article] [PubMed] [Google Scholar]
- [27].Sauer AM, Slooter AJ, Veldhuijzen DS, et al. Intraoperative dexamethasone and delirium after cardiac surgery: a randomized clinical trial. Anesth Analg 2014;119:1046–52. [DOI] [PubMed] [Google Scholar]
- [28].Whitlock RP, Devereaux PJ, Teoh KH, et al. Methylprednisolone in patients undergoing cardiopulmonary bypass (SIRS): a randomised, double-blind, placebo-controlled trial. Lancet 2015;386:1243–53. [DOI] [PubMed] [Google Scholar]
- [29].Balkanay OO, Goksedef D, Omeroglu SN, et al. The dose-related effects of dexmedetomidine on renal functions and serum neutrophil gelatinase-associated lipocalin values after coronary artery bypass grafting: a randomized, triple-blind, placebo-controlled study. Interact Cardiovasc Thorac Surg 2015;20:209–14. [DOI] [PubMed] [Google Scholar]
- [30].Djaiani G, Silverton N, Fedorko L, et al. Dexmedetomidine versus propofol sedation reduces delirium after cardiac surgery: a randomized controlled trial. Anesthesiology 2016;124:362–8. [DOI] [PubMed] [Google Scholar]
- [31].Grover S, Kate N. Assessment scales for delirium: a review. World J Psychiatry 2012;2:58–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Inouye SK. Delirium in older persons. N Engl J Med 2006;354:1157–65. [DOI] [PubMed] [Google Scholar]
- [33].Ely EW, Shintani A, Truman B, et al. Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. JAMA 2004;291:1753–62. [DOI] [PubMed] [Google Scholar]
- [34].Martin BJ, Arora RC. Delirium and cardiac surgery: progress: and more questions. Cri Care 2013;17:140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Geng J, Qian J, Cheng H, et al. The influence of perioperative dexmedetomidine on patients undergoing cardiac surgery: a meta-analysis. PLoS One 2016;11:e0152829. [DOI] [PMC free article] [PubMed] [Google Scholar]


