Abstract
This study aims to evaluate the application of color Doppler ultrasound in monitoring the complications of autologous arteriovenous fistula in hemodialysis patients.
Patients with maintenance hemodialysis who underwent autologous arteriovenous fistula were enrolled in this cross-sectional study. Ultrasound was used to detect fistula complications (stenosis and thrombosis), brachial artery diameter, and hemodynamic parameters. The ultrasound parameters were analyzed and screened to identify the most important indicator for monitoring complications.
In all, 89 patients were included. Ultrasound showed 72 cases (80.90%) had normal fistula structure, and 17 cases (19.10%) had complications. The diameter, time-averaged mean velocity, flow volume, and diastolic peak velocity of brachial artery in complication group were significantly lower than those of noncomplication group (P < .05). The brachial artery pulsatility index and resistance index of complication group were significantly higher than those of noncomplication group (P < .05). There was no significant difference in peak flow velocity between complication and noncomplication group (P > .05). Indicators showed statistical significance were grouped based on quantiles. The incidence of complications was higher when the brachial artery diameter was ≤5.40 mm, or brachial artery flow was ≤460 mL/ min, or brachial artery pulsatility index was >1.04, or brachial artery resistance index was >0.60.
Ultrasound monitoring of brachial artery diameter and hemodynamic parameters can help early detection of fistula complications. When the brachial artery diameter was ≤5.40 mm, or brachial artery flow was ≤460 mL/min, or brachial artery pulsatility index was >1.04, or brachial artery resistance index >0.60, stenosis or thrombosis should be checked to prevent fistula failure.
Keywords: autologous arteriovenous fistula, brachial artery, color Doppler ultrasound, hemodialysis
1. Introduction
Chronic kidney disease (CKD) is a major disease threatening human health. In 2013, it was estimated that the prevalence of global CKD is about 8% to 16%.[1] CKD will eventually develop to end-stage renal disease that requires renal replacement therapy.[2] Hemodialysis is the most commonly used method of renal replacement therapy.[3] The Shanghai dialysis registration report in 2014[4] showed that there were 1013 new hemodialysis patients in 2013 and 9170 patients hemodialysis patients by the end of 2013. About 2936 patients received peritoneal dialysis, accounting for 1/3 of all hemodialysis patients. High-quality vascular access (with sufficient blood flow, long lifespan, and low incidence of complications) is the most basic and most important issue in hemodialysis.
The 3 commonly used vascular accesses are autonomic arteriovenous fistula (AVF), synthetic arteriovenous graft (AVG), and central venous catheter (CVC). AVF is recognized as the best vascular access due to long lifespan, low incidence of cardiovascular events, and complications.[5–8] The K/DOQI guideline suggests that AVF should be used in at least 65% patients and CVC should be used in less than 10% patients.[7] The US Renal Data system showed that by the end of 2014, approximately 63.1% used AVF, 18.1% used AVG, and 18.8% used CVC.[3] Thrombosis and stenosis are closed related with AVF, and they will increase patients’ economic burden and lower their quality of life, which are the main causes for AVF failure.[9] In a prospective 2-year longitudinal study, 237 (32.3%) of 734 patients had AVF stenosis or thrombosis, leading to AVF failure.[10]
With proper monitor, thrombosis and stenosis can be early detected and timely treated, extending the life of fistula and increasing patient's survival rate.[11] Color Doppler ultrasound is used widely in clinic[12–15] and is recommended as the best way to monitor AVF due to its noninvasiveness, no radioactive damage, repeated usage, low price, and real-time access to AVF anatomical and hemodynamic information.[16,17] The brachial artery is straighter with larger diameter and the blood flow is more accurately measured by Doppler ultrasound. Thus, the brachial artery is considered as the best measurement site for ultrasound monitoring of AVF.[18] There are no uniform ultrasound parameters for monitoring AVF.[7,19] Flow monitoring can reduce the incidence of thrombosis or stenosis. However, the extent of flow intervention in AVF is under debate.[20,21] The flow ultrasound parameters other than hemodynamics are also lacking.
This study aims to monitor AVF by color Doppler ultrasound. The relationship between the complications (stenosis and thrombosis) and brachial artery parameters on ultrasound were analyzed. The ultrasound parameters with high clinical value were screened.
2. Materials and methods
2.1. Patients
Patients who underwent maintenance hemodialysis by AVF in our hospital from July, 2016 to December, 2016 were included. The inclusion criteria were as follows: age >18 years; received AVF for maintenance hemodialysis; AVF were stable for more than 3 months; AVF using forearm radial artery–head vein anastomosis. Patients were excluded if they had severe heart failure or arrhythmia; could not maintain limb with AVF stability during ultrasound; had vascular abnormalities (bifurcation of radial or ulnar artery from the axillary artery). Prior written and informed consent was obtained from every patient, and the study was approved by the ethics review board of Huashan Hospital, Fudan University.
2.2. Color Doppler ultrasound
The HI VISION Avius L color Doppler ultrasound (Hitachi Science and Systems, Tokyo, Japan) was used with linear array probe and frequency of 8 to 12 MHz. All patients underwent ultrasound at 1 hour before dialysis. The patient was in sitting position with elbow pad to stabilize the upper limb. The examination site was fully exposed, and the examination was cancelled if the skin was damaged or with puncture bleeding. The brachial artery of the upper arm of the AVF side was measured. The brachial artery diameter (D), time-averaged mean velocity (Vm), flow volume (FV), peak systolic velocity (PSV), end-diastolic velocity (EDV), pulsatility index (PI), and resistance index (RI) were measured. The anastomosed blood vessels and way of anastomosis of AVF were determined. The inflow of AVF radial artery, anastomosis, and outflow of the head vein were detected for AVF stenosis and thrombosis. Ultrasound examination had to be carried out carefully. The probe had to be kept vertical to the skin surface. Sufficient amount of coupling agent was applied with gentle touch to avoid pressures on blood vessels, especially the veins. The entire vascular cavity was covered to avoid neglecting low-speed blood flow along the vascular wall. Doppler angle was set at ≤60° (Fig. 1).
Figure 1.
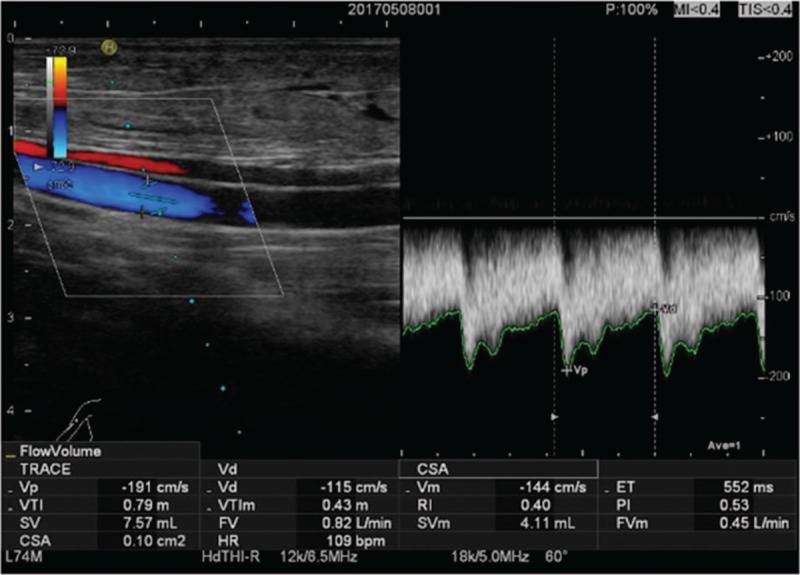
The ultrasound parameters of brachial artery. The sampling area covered the entire vascular lumen to avoid neglecting the low-speed blood flow along the vascular wall with Doppler angle ≤60°.
2.3. Thrombosis and stenosis
The outcome indicator of AVF was thrombosis or stenosis. According to previous reports,[8,22] fistula stenosis was defined as anastomotic diameter <2.0 mm, and the diameter of the narrow area was 50% less than the inner diameter of the adjacent normal section. Thrombosis was diagnosed as hypoechoic or hyperechoic in part or all of vascular cavity, and filling defect was detected by color Doppler ultrasound.
2.4. Statistical analysis
Data were analyzed using SPSS22.0 software. Data of normal distribution were expressed as mean ± standard deviation. The t test was used for group comparison. The non-normal distributed data were expressed as median, and rank-sum test was used for group comparison. Categorical data were expressed as frequency and percentage, and chi-square test was used. P < .05 was considered statistically significant. When there are multiple pair-wise comparisons, it is necessary to reduce the significant difference (α′ = α/N, N = K (K − 1)/2, α = 0.05, K = number of group) to avoid increasing the error of type I.[23]
3. Results
3.1. Clinical data of patients
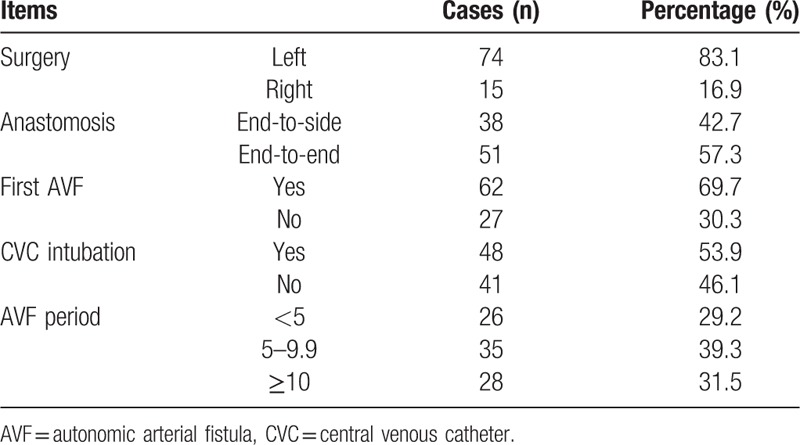
According to the inclusion criteria, 97 patients were initially included. Then, 8 patients, including 2 patients with arrhythmia, 2 patients with autologous arteriovenous fistula and involuntary tremor, and 4 patients with abnormal vessel shape, were excluded according to the exclusion criteria. Finally, 89 patients were recruited, including 45 males and 44 females, aging from 32 to 84 years with an average age of 62.77 ± 10.80 years (Table 1). The dialysis period ranged from 0.62 to 25.4 years, with an average period of 9.31 ± 5.96 years. Patients with more than 10 years of dialysis accounted for 41.6%. There were 35 cases of chronic glomerulonephritis, 11 cases of diabetic nephropathy, 20 cases of hypertension nephropathy, 8 cases of polycystic kidney disease, and 14 cases of other diseases (such as pyelonephritis and lupus nephritis). As shown in Table 2, there were 74 cases of left AVF; 15 cases of right AVF; 51 cases of end-to-end anastomosis; 38 cases of end-to-side anastomosis; 62 patients used AVF for the first time; 48 cases of patients had history of CVC intubation; and 28 patients used AVF for more than 10 years.
Table 1.
Patient basic information.
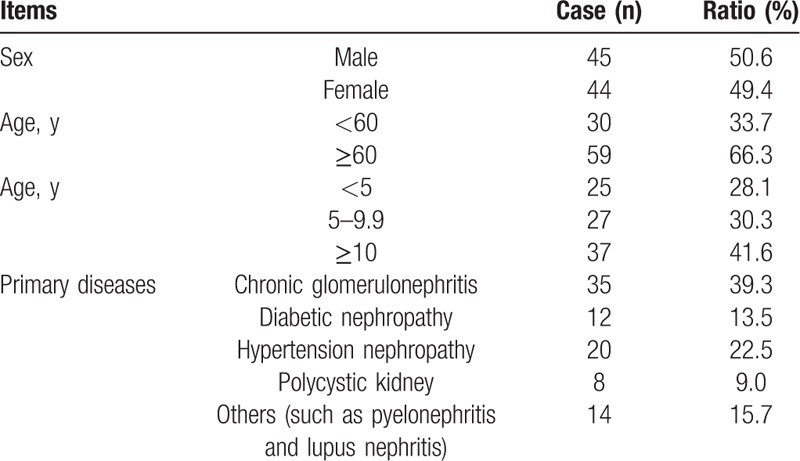
Table 2.
AVF characteristics.
3.2. AVF complications and ultrasound parameters
As shown in Table 3, of the 89 patients, 17 cases (19.1%) had complications (stenosis or thrombosis), 17 cases (19.1%) had stenosis (including 9 cases of anastomotic stenosis and 8 cases of venous stenosis), 2 cases (2.2%) had thrombosis (both in the vein, leading to thrombotic stenosis), and 2 cases (2.2%) had both stenosis and thrombosis (Fig. 2 and Fig. 3).
Table 3.
Autonomic arteriovenous fistula complications and ultrasound parameters of brachial artery.
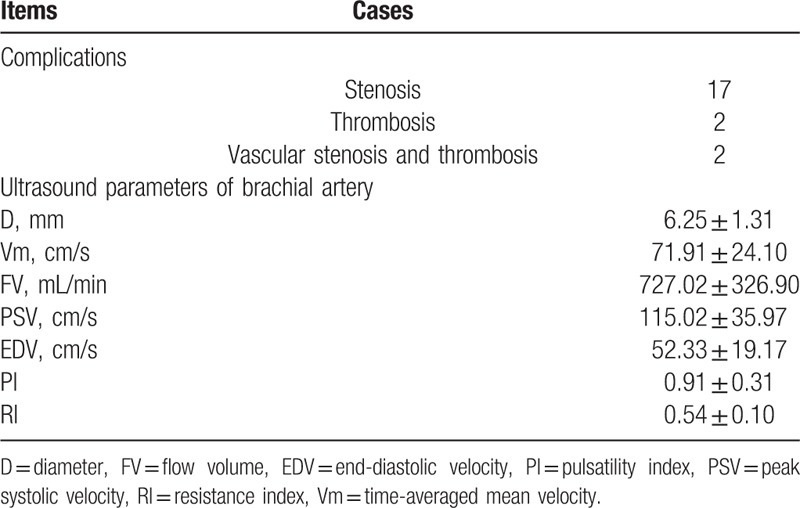
Figure 2.
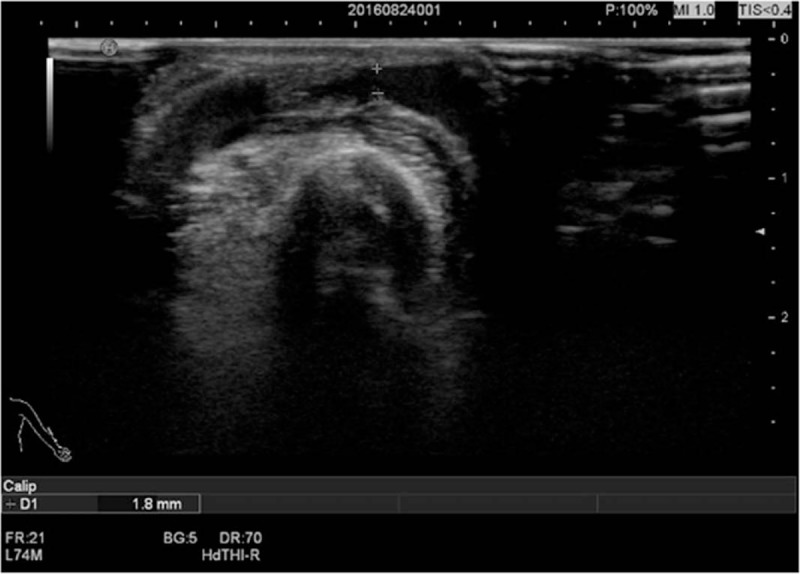
The anastomotic stenosis with anastomosis of 1.8 mm (<2 mm).
Figure 3.
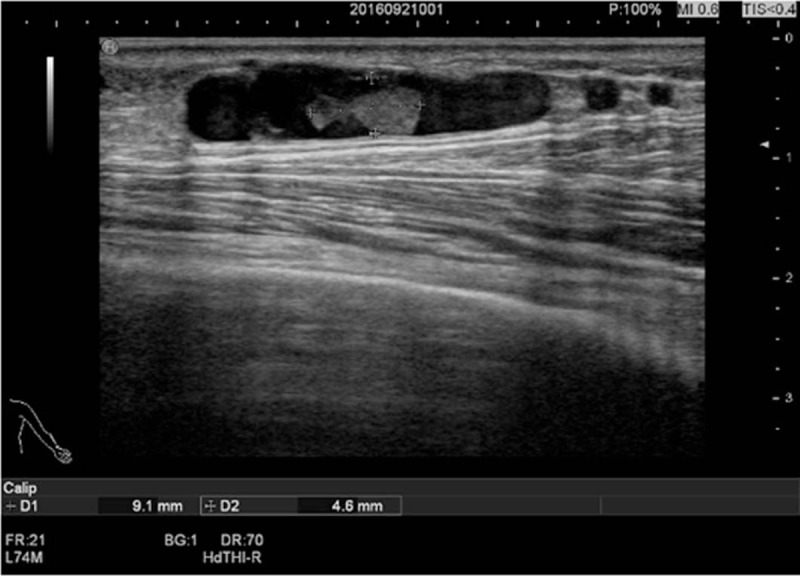
Head vein thrombosis at 14 cm from the anastomosis (longitudinal length of 9.1 mm, width of 4.6 mm).
The mean brachial artery AVF D ranged from 3.70 to 10.30 mm, with an average of 6.25 ± 1.31 mm; the Vm ranged from 26.40 to 163.00 cm/s, with an average of 71.91 ± 24.10 cm/s; FV ranged from 140.00 to 1780 mL/min, with an average of 727.02 ± 326.90 mL/min; PSV ranged from 44.30 to 231.00 cm/s, with an average of 115.02 ± 35.97 cm/s; EDV ranged from 16.70 to 136.00 cm/s, with an average of 52.33 ± 19.17 cm/s; PI ranged from 0.31 to 2.15, with an average of 0.91 ± 0.31; and RI ranged from 0.27 to 0.79, with an average of 0.54 ± 0.10.
3.3. Relationship between AVF complications and ultrasound parameters
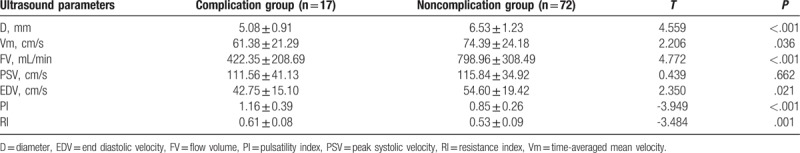
To determine the relationship between AVF complications and ultrasound parameters, their correlation was analyzed. According to the presence of complications, patients were divided into complication group (n = 17) and noncomplication group (n = 72). As shown in Table 4, the levels of D, Vm, FV, and EDV were significantly lower in the complication group than those in the noncomplication group (P < .05). PI and RI were significantly higher in the complication group than those in the noncomplication group (P < .05). There was no significant difference in PSV between complication and noncomplication groups (P > .05). It indicates that brachial artery D, Vm, FV, EDV, PI, and RI were significantly related to AVF complications.
Table 4.
Comparison of ultrasound parameters between complication group and noncomplication group.
3.4. Cut-off points of ultrasound parameters on AVF complications evaluation
To identify the cut-off points of ultrasound parameters, they were grouped by quartiles. In this study, K = 4, therefore P < .0083 was considered statistically significant to avoid type I error. The incidence of complications in each group is shown in Table 5.
Table 5.
Complication rate.
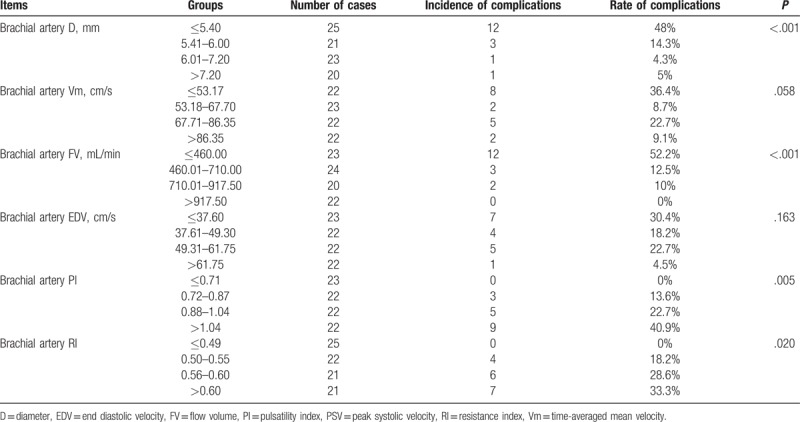
Brachial artery D (range 3.70–10.30 mm) were grouped as: group A: ≤5.40, group B: 5.41 to 6.00, group C: 6.01 to 7.20, and group D: >7.20. It was found that the incidence of AVF complications significantly increased with the increase of brachial artery D (P < .05). There were significant differences between group A and group C, and between group A and group D (P < .0083). When the brachial artery D was ≤5.40 mm, there were high complication rates.
The brachial artery Vm (range 26.40–163.00 cm/s) was grouped as group A: ≤53.17 cm/s, group B: 53.18 to 67.70 cm/s, group C: 67.71 to 86.35 cm/s, and group D: >86.35 cm/s. There were no significant differences among the 4 groups (P > .05).
The brachial artery FV (range 140.00–1780.00 mL/min) was grouped as group A: ≤460.00 mL/min, group B: 460.01 to 710.00 mL/min, group C: 710.01 to 917.50 mL/min, and group D: >917.50 mL/min. The incidence of AVF complications was significantly decreased with the increase of brachial artery FV (P < .05). There was significant difference between group A and group B, group A and group C, and group A and group D (P < .0083). Therefore, when the brachial artery FV was ≤460 mL/min, the complication rate was high.
The brachial artery EDV (range 16.70–136.00 cm/s) was grouped as group A: ≤37.60 cm/s, group B: 37.61 to 49.30 cm/s, group C: 49.31 to 61.75 cm/s, and group D: >61.75 cm/s. There was no significant difference among the groups (P > .05).
The brachial artery PI (range 0.31–2.15) was grouped as group A: ≤0.71, group B: 0.72 to 0.87, group C: 0.88 to 1.04, and group D: >1.04. AVF complications increased with PI (P < .05). There were significant differences between group A and group D (P < .0083). Therefore, when brachial artery PI was >1.04, the complication incidence rate was high.
The brachial artery RI (range 0.27–0.79) was grouped as group A: ≤0.49, group B: 0.50 to 0.55, group C: 0.56 to 0.60, and group D: >0.60. AVF complications increased with RI (P < .05). There was significant difference between group A and group D (P < .0083). Therefore, when brachial artery RI was >0.60, the complication rate was high.
In conclusion, the cut-off points of brachial artery D, FV, PI, and RI are identified. It showed that when the brachial artery D was ≤5.40 mm, or FV was ≤460 mL/min, or PI was >1.04, or RI was >0.60, the complication rate was high.
4. Discussion
Autonomic arteriovenous fistula is the first-line vascular pathway for dialysis patients, and ultrasound is the recommended AVF method.[7,8] The K/DOQI guideline recommends measuring the inflow of the infarcted blood vessels, rather than the anastomotic and outflow tract vessels, which may be because that the anastomotic blood flow is not laminar. Apart from this, the head vein wall is thin and superficial that is easily pressed by the probe, and dialysis puncture for a long time is easy to promote tumor-like expansion that is difficult for head vein measurement. The radial artery may underestimate the flow of AVF and ignore the ulnar artery flow into AVF at the distal end of anastomosis.[24] In this study, brachial artery was selected to monitor AVF.
We analyzed the relationship between the complications (stenosis and thrombosis) and the ultrasound parameters of AVF brachial artery. The result showed that D, Vm, FV, EDV, PI, and RI of brachial artery were significantly correlated with AVF complications, and cut-off points of each indicator were identified to provide the evidence for the early detection of AVF complications.
Apart from this, we found that the brachial artery D in the complication group was significantly lower than that in the noncomplication group. The incidence of AVF complications decreased as brachial artery D increased. When the brachial artery D was ≤5.40 mm, the complication incidence was high. This result indicates that the brachial artery D has significant individual differences; however, when it was ≤5.40 mm, AVF stenosis and thrombosis should be checked. The brachial artery Vm of the complication group was significantly lower than that of the noncomplication group. The incidence of complications decreased as the brachial artery Vm increased. However the quartile method found no boundary value, which may be due to small sample size. For AVF brachial artery Vm, follow-up with large sample size should be performed to determine the appropriate cut-offs. Additionally, we found that brachial artery FV in the complication group was significantly lower than that in the noncomplication group. The incidence of AVF complications decreased with FV increase. When the brachial artery FV was ≤460 mL/min, the complication incidence was high. Some reported that AVF and AVG flow should be between 300 and 400 mL/min to 3000 mL/min, most as 600 to 1500 mL/min.[18] A Chinese study[22] showed that brachial artery FV had large individual differences, including 46 cases of AVF brachial artery FV ranging from 203 to 2386 mL/min. The above differences could be resulted from differences of ethnicity.
It is suggested that[25] the incidence of stenosis was great when the flow rate was <650 mL/min. It is also reported that[11] the incidence of thrombosis was great when the flow rate was <500 mL/min or decrease >25%. The cut-off point was slightly lower in this study, probably due to the overall lower AVF flow of Chinese patients.[18,26] This study suggests that when the brachial artery FV is ≤460 mL/min, AVF stenosis and thrombosis should be checked. On the contrary, high brachial artery FV may increase heart load. It is recommended that cardiac ultrasound should also be used to determine whether interventions (such as anastomosis) should be used when brachial artery FV is high.
In this study, we also found that the brachial artery EDV in complication group was significantly lower than that in the noncomplication group. The incidence of complications increased as brachial artery EDV decreased. However, the cut-off points were unable to identify, possibly due to limited sample size. Future studies with larger sample size should be used to determine the cut-offs. Brachial artery PI was significantly higher in the complication group than in the noncomplication group. The incidence of AVF complication increased as brachial artery PI increased. When brachial artery PI value is >1.04, AVF stenosis and thrombosis should be checked. The brachial artery RI in the complication group was significantly higher than that in the noncomplication group. The incidence of AVF complication increased as brachial artery RI increased. When the brachial artery RI is >0.60, complication is more likely to happen. For brachial artery RI, Koseoglu et al[27] showed that brachial artery RI (measured 3–4 cm above elbow) was a predictor of AVF failure. Moreno Sanchez et al[19] reported that stenosis and thrombosis could lead to increased RI in artery inflow. Our study was consistent with these studies, demonstrating that with abnormal AVF, brachial artery RI significantly increases. Our study also recommend that, for AVF radial artery-head vein anastomosis, when brachial artery RI is >0.60, AVF stenosis and thrombosis should be checked.
This study is limited in the small sample size. Apart from this, the subjects were from the same dialysis center. Thus, the conclusion might be limited.
5. Conclusions
In conclusion, D, FV, PI, and RI of brachial artery were significantly related with AVF complications. This study showed that when the brachial artery D ≤5.40 mm, or brachial artery FV ≤460 mL/min, or brachial artery PI >1.04, or brachial artery RI >0.60, the incidence of complications (stenosis and thrombosis) may increase. Therefore, brachial artery diameter and hemodynamic parameters are helpful for the early detection of fistula stenosis and thrombosis. For brachial artery D ≤5.40 mm, brachial artery FV ≤460 mL/min, brachial artery PI >1.04, and brachial artery RI >0.60, stenosis and thrombosis should be checked for early detection of complications.
Author contributions
Data curation: Chong Ren, Yanpei Cao.
Funding acquisition: Yanpei Cao.
Investigation: Chong Ren, Wenwen Lu.
Methodology: Chong Ren.
Project administration: Jing Chen.
Resources: Yong Wang, Bihong Huang.
Software: Xiaoli Yang.
Validation: Jing Chen.
Writing – original draft: Chong Ren.
Writing – review & editing: Yanpei Cao.
Footnotes
Abbreviations: AVF = autonomic arteriovenous fistula, AVG = arteriovenous graft, CKD = chronic kidney disease, CVC = central venous catheter, EDV = end-diastolic velocity, FV = flow volume, PI = pulsatility index, PSV = peak systolic velocity, RI = resistance index.
Funding: This study is supported by the Shanghai Municipal Commission of Health and Family Planning Research Fund [No: 201640126 and No: 201540085] and the Fudan University Research Fund [No: FNF201529 and FNF201610].
The authors declared no conflict of interest.
References
- [1].Jha V, Garcia-Garcia G, Iseki K, et al. Chronic kidney disease: global dimension and perspectives. Lancet 2013;382:260–72. [DOI] [PubMed] [Google Scholar]
- [2].Schroeder EB, Yang X, Thorp ML, et al. Predicting 5-year risk of RRT in stage 3 or 4 CKD: development and external validation. Clin J Am Soc Nephrol 2017;12:87–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].USRDS. Annual Data Report. Washington: USRDS Coordinating Center; 2016. [Google Scholar]
- [4].Center SHQC. Shanghai dialysis registration report in 2014. Shanghai: Shanghai Hemodialysis Quality Control Center; 2014. [Google Scholar]
- [5].Ocak G, Rotmans JI, Vossen CY, et al. Type of arteriovenous vascular access and association with patency and mortality. BMC Nephrol 2013;14:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Pisoni RL, Arrington CJ, Albert JM, et al. Facility hemodialysis vascular access use and mortality in countries participating in DOPPS: an instrumental variable analysis. Am J Kidney Dis 2009;53:475–91. [DOI] [PubMed] [Google Scholar]
- [7].Group VAW. NKF-KDOQI clinical practice guidelines for vascular access. Am J Kidney Dis 2006;48 suppl 1:S1–322. [DOI] [PubMed] [Google Scholar]
- [8].Wang YZ, Ye CY, Jin QZ. China expert consensus on hemodialysis vascular access. Zhong Guo Xue Ye Jing Hua 2014;13:549–58. [Google Scholar]
- [9].Al-Jaishi AA, Liu AR, Lok CE, et al. Complications of the arteriovenous fistula: a systematic review. J Am Soc Nephrol 2017;28:1839–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Premuzic V, Hudolin T, Pasini J, et al. Hypoproteinemia as a prognostic risk factor for arteriovenous fistula failure. Hemodial Int 2018;22:37–44. [DOI] [PubMed] [Google Scholar]
- [11].Aragoncillo I, Amezquita Y, Caldes S, et al. The impact of access blood flow surveillance on reduction of thrombosis in native arteriovenous fistula: a randomized clinical trial. J Vasc Access 2016;17:13–9. [DOI] [PubMed] [Google Scholar]
- [12].Gao Z, Li Y, Sun Y, et al. Motion tracking of the carotid artery wall from ultrasound image sequences: a nonlinear state-space approach. IEEE Trans Med Imaging 2018;37:273–83. [DOI] [PubMed] [Google Scholar]
- [13].Guo M. A Rod-like Acoustic Radiation Force in Ultrasound-based Elastography: A Simulation Study. Cham: The International Conference on Health Informatics. Springer; 2014. [Google Scholar]
- [14].Hu Z1, Zhang H, Yuan J, et al. An H strategy for strain estimation in ultrasound elastography using biomechanical modeling constraint. PloS One 2013;8:e73093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Cao K, Mills DM, Thiele RG, et al. Toward quantitative assessment of rheumatoid arthritis using volumetric ultrasound. IEEE Trans Biomed Eng 2016;63:449–58. [DOI] [PubMed] [Google Scholar]
- [16].Guedes Marques M, Ibeas J, Botelho C, et al. Doppler ultrasound: a powerful tool for vascular access surveillance. Semin Dial 2015;28:206–10. [DOI] [PubMed] [Google Scholar]
- [17].Visciano B, Riccio E, De Falco V, et al. Complications of native arteriovenous fistula: the role of color Doppler ultrasonography. Ther Apheresis Dial 2014;18:155–61. [DOI] [PubMed] [Google Scholar]
- [18].Thrush A, Hartshorne T. Vascular Ultrasound: How, Why, and When. 3rd ed. London: Churchill Livingstone; 2010:305–311. [Google Scholar]
- [19].Moreno Sanchez T, Martin Hervas C, Sola Martinez E, et al. Value of doppler ultrasonography in the study of hemodialysis peripheral vascular access dysfunction. Radiologia 2014;56:420–8. [DOI] [PubMed] [Google Scholar]
- [20].Tonelli M, James M, Wiebe N, et al. Ultrasound monitoring to detect access stenosis in hemodialysis patients: a systematic review. Am J Kidney Dis 2008;51:630–40. [DOI] [PubMed] [Google Scholar]
- [21].Tessitore N, Bedogna V, Poli A, et al. Should current criteria for detecting and repairing arteriovenous fistula stenosis be reconsidered? Interim analysis of a randomized controlled trial. Nephrol Dial Transplant 2014;29:179–87. [DOI] [PubMed] [Google Scholar]
- [22].Zhu YL, Ding H, Fan PL, et al. Blood flow and hemodynamic evaluation on autogenous arteriovenous fistulas for hemodialysis access with color Doppler ultrasound. Zhong Guo Chao Sheng Yi Xue Za Zhi 2014;30:824–6. [Google Scholar]
- [23].Huang SP. The method of multiple comparisons of rate. Xu Zhou Yi Xue Yuan Xue Bao 2002;4:291–4. [Google Scholar]
- [24].Wiese P, Nonnast-Daniel B. Colour Doppler ultrasound in dialysis access. Nephrol Dial Transplant 2004;19:1956–63. [DOI] [PubMed] [Google Scholar]
- [25].Tessitore N, Bedogna V, Melilli E, et al. In search of an optimal bedside screening program for arteriovenous fistula stenosis. Clin J Am Soc Nephrol 2011;6:819–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Gao HG, Su GM, Zhu L, et al. Application value of ultrasound in monitoring the native arteriovenous fistula. Yi Xue Ying Xiang Xue Za Zhi 2010. 592–5. [Google Scholar]
- [27].Koseoglu K, Akar H, Cildag B, et al. Resistive index measurement of native hemodialysis arteriovenous fistula feeding artery as a predictor for fistula dysfunction. ASAIO J 2004;50:577–80. [DOI] [PubMed] [Google Scholar]


