Abstract
Aims:
To investigate the metabolic risk factors according to the degree of obesity in Korean adolescents.
Methods:
Among 7197 subjects aged 10–18 years who participated in the 2007-2014 K-NHANES, 1326 adolescents (M = 744, F = 582) with age and sex specific body mass index (BMI) ≥85th percentile were included. These adolescents with obesity were classified as: overweight, obesity, severe obesity, and extreme severe obesity. For assessing central obesity, the subjects were further-classified as: normal waist obese, abdominal obesity I, abdominal obesity II and abdominal obesity III.
Results:
The prevalence of overweight, obesity, severe obesity and extreme severe obesity were 5.6%, 6.2%, 5.9% and 0.9% in Korean adolescents. With increasing levels of obese category, the incidence of metabolic risk factors such as HDL-C<40 mg/dL or <50 mg/dL in girls older than 16 years-old (20.2%, 18.5%, 34.4%, 43.6%, P<0.0001), TG ≥150 mg/dL (15.3%, 16.7%, 26.5%, 30.9%, P<0.003), HbA1C ≥ 5.8% (12.8%, 13.5%, 21.9%, 42.2%, P<0.006), SBP ≥ 130 mg/dL (3.5%, 6.4%, 8.1%, 19.5%, P<0.003) significantly increased. With increasing levels of central obese category, the incidence of metabolic risk factors such as HDL-C<40 mg/dL or <50 mg/dL in girls older than 16 years-old (20.2%, 26.2%, 37.9%, 35.7%, P<0.0007), TG ≥ 150 mg/dL (16.1%, 21.2%, 25.8%, 29.8%, P<0.004), glucose ≥ 100 mg/dL (7.7%, 7.3%, 11.7%, 17.4%, P<0.009) and SBP ≥ 130 mg/dL (5.1%, 7.1%, 3.0%, 13.9%, P<0.002) significantly increased.
Conclusion:
Adolescents with severe obesity have more metabolic risk factors compared to adolescents with less severe degree of obesity.
Keywords: Severe obesity, Adolescents, Morbidity
1. Introduction
Severe obesity in adolescents has become an important global public health issue. Despite recent data suggesting that the rate of increasing obesity in children and adolescents has slowed and the overall prevalence has possibly begun to plateau, a worrisome trend has emerged in the form of severe pediatric obesity [1]. Skinner et al. have reported an upward trend in severe obesity among children in the United States [2]. Some reports have forecasted a 130% increase in severe obesity prevalence over the next two decades [3].
Childhood obesity is a major health concern and has prompted increasing attention to potential long-term health issues. The risk of death from all causes including cardiovascular disease, cancer, or other diseases among severely obese adults is twice that of moderately obese adults [4]. Numerous previous reports have been presented epidemiological evidences of tracking of childhood obesity into adulthood. The tracking of childhood obesity into adulthood has been found in various filed of adulthood obesity. Childhood obesity has been previously reported to be an important predictor of adult obesity [5]. Some reports found substantial tracking of childhood obesity to adult cardiovascular disease [6]. A child with severe obesity is more likely to be an obese adult compared to those with moderate juvenile obesity [7]. Especially, extreme severe pediatric obesity tracks strongly into adulthood [5,8–10]. Childhood obesity has also been reported as a predictor a broad range of detrimental health consequences in adults with independence of adult weight [11].
The prevalence of metabolic syndrome is high among children with obesity and increases with worsening obesity [12]. Approximately 60% of adolescents with severe obesity have two or more risk factors of cardiovascular disease [9]. Tracking from metabolic syndrome of adolescents with obesity to cardiovascular disease, metabolic syndrome or even mortality in adults has been reported [6,11]. Therefore, medical care and management of the current morbidity in adolescents with severe obesity are urgent. However, little information is available on the characteristics of metabolic risk factors or the current health state in Korean children with severe obesity. Therefore, we conducted a cross-sectional study based on data obtained in the 2007-2014 Korean National Health and Nutrition Examination Surveys (KNHANES) to explore the associations between metabolic risk factors and severe obesity in Korean adolescents.
2. Methods
This study was based on data obtained from the fifth Korean National Health and Nutrition Examination Survey conducted in 2007-2014 by the Korean Ministry of Health and Welfare. KNHANES surveys are conducted annually using a rolling sampling design that involves a complex, stratified, multistage, probability-cluster survey of a representative sample of the noninstitutionalized civilian population in South Korea. All individuals are randomly selected. Data were collected in a variety of ways, including household interviews, physical examinations, laboratory tests and nutritional status assessments. All survey protocols were approved by the Korea Centers for Disease Control and Prevention Institutional Review Board. Written informed consent was obtained from all participants before the survey began. The K-NHANES data are publicly available with no charge [13].
2.1. Selection of study populations
Among 7197 subjects aged 10–18 years with available anthropometric data who participated in the 2007-2014 K-NHANES, 1326 participants (male = 744, female = 582) with age and sex specific body mass index (BMI) ≥85th percentile were included in the present study. Among 7197 subjects, 5871 participants with age and sex specific body mass index (BMI) <85th percentile were excluded.
2.2. Anthropometric and laboratory measurements
Height was measured using a stadiometer (Seca, Hamburg, Germany), and weight was measured with a balance beam scale (GL-6000-20, CASKOREA, Korea) while participants were wearing a standard gown. Waist circumference (WC) was measured to the nearest 0.1 cm at the end of normal expiration, measuring from the narrowest point between the midline of the most lateral border of the right and left iliac crest. Monthly family income indicates monthly equalized family income and was calculated by dividing total family income by the square root of the number of household members. In K-NHANES, income was classified into quartiles to determine monthly household income level (1: low, 2: middle low, 3: middle high, 4: high).
Fasting plasma concentrations including glucose (n = 1123), glycosylated hemoglobin (HbA1c) (n = 488), total cholesterol (TC, n = 1128), triglyceride (TG, n = 1128), high density lipoprotein-cholesterol (HDL-C, n = 1128) low density lipoprotein-cholesterol (LDL-C, n = 1124) of the subjects were measured enzymatically using a Hitachi 747 chemistry analyzer (Daiichi, Tokyo, Japan) after subjects performed a minimum 8-hour overnight fast. Systolic blood pressure (SBP) and diastolic blood pressure (DBP) were measured using a mercury sphygmomanometer [Baumanometer Wall Unit 33(0850), W. A. Baum, New York, USA] according to the standardized protocol by trained personnel. Appropriate size of BP cuff was applied based on participants’ arm circumference. BP was measured three times after sitting at least 5 minutes. The mean value of the last two readings was used for the analysis.
2.3. Study criteria
In this study, adolescents with obesity were classified by increasing levels of BMI percentile according to the following categories: overweight (85th ≤ BMI < 95th percentile), obesity (95th ≤ BMI<120% of 95th BMI), severe obesity (≥ 120% of 95th BMI or BMI 35, whichever was lower), and extreme severe obesity (≥ 140% of 95th BMI or BMI 40, whichever was lower) using the 2007 Korea Growth Charts [1,14,15]. For assessing central obesity, the subjects were further-classified by increasing levels of WC percentile according to the following categories: normal waist obese (WC<90th percentile), abdominal obesity I (90th ≤ WC <95th), abdominal obesity II (95th ≤ WC <97th) and abdominal obesity III (97th ≤ WC) using the 2007 Korea Growth Charts [14].
2.4. Definitions of abnormal metabolic risk factors
We used standard cut off values of the criteria for abnormal metabolic risk factor in adolescents as follows: TC ≥ 200 mg/dL, HDL-C < 40 mg/dL or < 50 mg/dL in girls older than 16 years, LDL-C ≥ 130 mg/dL, TG ≥ 150 mg/dL, glucose ≥ 100 mg/dL, HbA1C ≥ 5.8%, SBP ≥ 130 mmHg, and DBP ≥ 85 mmHg [16,17].
2.5. Statistical analyses
All statistical analyses were performed using SAS Version 9.3 (SAS Institute Inc., Cary, NC). Sampling weights were incorporated to produce valid population estimates that accounted for the complex survey design of the K-NHANES. Data were presented as geometric mean (95% confidence interval, CI) for continuous variables and frequency percentage for categorical variables. The characteristics and laboratory data of body composition among the degree of obesity were compared using ANCOVA with Bonferroni corrections to assess the significant pairwise differences for continuous measures and chi-squared tests for categorical measures. Multivariate logistic regression models were used to compare the adjusted odds ratio (OR) and 95% CI of metabolic risk factors among body composition groups after adjusting for potentially confounding variables. P values less than 0.05 were considered as statistically significant.
3. Results
(1). Characteristics and anthropometric data of 10-18-year-old adolescents with obesity.
Among 1326 adolescents (male = 744, female = 582) with age and sex specific BMI ≥ 85th percentile, the weighted prevalence of overweight, obesity, severe obesity, and extreme severe obesity were 5.6% (413/7197), 6.2% (449/7197), 5.9% (410/7197), and 0.9% (56/7197), respectively. Geometric means of age, current HT (height), current HT standard deviation score (SDS), current WT (weight), current WT SDS, current BMI, current BMI SDS, and current WC are presented in Table 1.
Table 1.
Characteristics and anthropometric data of 10-18-year-old adolescents with obesity.
| Number or Mean (95% CI) | |
|---|---|
| Number, weighted prevalence | 1326/7197, 18.6% (17.5-19.5) |
| Overweight | 413/7197, 5.6% (5.0-6.2) |
| Obesity | 447/7197, 6.2% (5.5-6.9) |
| Severe obesity | 410/7197, 5.9% (5.2-6.6) |
| Extreme severe obesity | 56/7197, 0.9% (0.6-1.1) |
| Age | 14.2 (14.0-14.3) |
| Sex | F=582, M=744 |
| current Height (cm) | 162.6 (161.9-163.4) |
| current Height _SDS | 0.4 (0.3-0.5) |
| current Weight (kg) | 70.2 (69.2-71.2) |
| current Weight _SDS | 1.48 (1.4-1.5) |
| current BMI (kg/m2) | 26.2 (26.0-26.4) |
| current BMI_SDS | 1.6 (1.57-1.63) |
| current WC (cm) | 82.8 (82.3-83.4) |
Data are presented as Number or Geometric means (95% CI)
Abbreviations: SDS, standard deviation score, BMI, body mass index; WC, Waist Circumference; overweight (85th ≤BMI<95th percentile); obesity (95th ≤BMI<120% of 95th BMI); severe obesity (≥120% of 95th BMI or BMI 35, whichever was lower); marked severe obesity (≥140% of 95th BMI or BMI 40, whichever was lower)
(2). The proportions based on obese or central obese category in total adolescents with obesity.
The proportion percentages of overweight (30.0%), obesity (33.4%), severe obesity (32.0%), and extreme severe obesity (4.7%) and the proportion percentages of normal waist obese (57.0%), abdominal obesity I (18.5%), abdominal obesity II (8.7%), and abdominal obesity III (15.8%) are checked in total adolescents with obesity.
Between male and female, there were no significant differences in the proportions of overweight, obesity, severe obesity, or extreme severe obesity when classified according to the degree of obesity. Between male and female, there were significant differences in the proportion percentages of normal waist obese (61.6 % vs. 51%), abdominal obesity I (14.8% vs. 23.2%), abdominal obesity II (8.4% vs. 9.2%), abdominal obesity III (15.2% vs. 16.6%) (P = 0.003). Between subjects younger than 14 years and those aged 15 years or older, there were significant differences in the proportions of overweight (31 % vs. 28.9 %), obesity (36.5% vs. 30%), severe obesity (29.9% vs. 34.2%), and extreme severe obesity (2.6 % vs. 6.9 %), respectively (P < 0.003). Between subjects younger than 14 years and those aged 15 years or older, there were also significant differences in the proportions of normal waist obese (59.3% vs. 54.4 %), abdominal obesity I (20.6% vs. 16.2%), abdominal obesity II (9 % vs. 8.5 %), and abdominal obesity III (11.1% vs. 20.9%) (P < 0.001)
(3) Anthropometry and metabolic risk factors according to obese or central obese category in 10-18 year-old adolescents with obesity
With increasing levels of obese category, the means of WT SDS (P < 0.001), BMI SDS (P < 0.001), TC (P < 0.001), TG (P < 0.001), HDL-C (P < 0.001), LDL-C (P < 0.001), SBP (P < 0.001), and HbA1c (P = 0.036) increased significantly after adjusting for age and sex. Among the four groups, the geometric means of HT SDS, DBP, and glucose showed no significant differences.
With increasing levels of central obese category, the geometric means (95% CI) of HT SDS (P < 0.001), WT SDS (P < 0.001), BMI SDS (P < 0.001), TC (P < 0.001), TG (P for trend < 0.001), HDL-C (P = 0.002), LDL-C (P < 0.001), and SBP (P = 0.016) increased significantly after adjusting for age and sex. Among the four groups, the geometric of DBP, glucose, HbA1c showed no significant differences (Table 2).
Table 2.
Anthropometry and metabolic risk factors according to category of obesity or central obesity in 10-18 year-old adolescents with obesity.
| Obese category | Central obese category | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Overweight (n=413) | Obesity (n=447) | Severe obesity (n=410) | Extreme severe obesity (n =56) | P-value | Normal waist obese (n=768) | Abdominal obesity I (n=256) | Abdominal obesity II (n=113) | Abdominal obesity III (n=189) | P-value | |
| Height_SDS | 0.4 (0.3, 0.5) | 0.4(0.3, 0.5) | 0.5(0.4, 0.6) | 0.4(−0.01, 0.7) | 0.721 | 0.3 (0.2, 0.4) | 0.5 (0.4, 0.7) a | 0.6 (0.3, 0.8) | 0.9 (0.7, 1.1)ab | <0.001 |
| Weight_SDS | 0.9(0.9, 1.0) | 1.2 (1.2, 1.3)a | 2.0 (1.9, 2.1)ab | 3.4 (3.2, 3.7)abc | <0.001 | 1.1 (1.0, 1.1) | 1.6 (1.5, 1.7) | 1.9 (1.8, 2.1)ab | 2.7 (2.6, 2.9)abc | <0.001 |
| BMI_SDS | 1.1 (1.1, 1.2) | 1.5 (1.4, 1.5)a | 2.0 (1.96, 2.02)ab | 2.8 (2.8, 2.9)abc | <0.001 | 1.3 (1.3, 1.4) | 1.7 (1.6, 1.7)a | 1.9 (1.8, 2.0)ab | 2.3 (2.2, 2.3)abc | <0.001 |
| TC (mg/dL) | 159.8 (156.4,163.3) | 163.9 (160.9,166.8) | 171.6 (167.5,175.6)ab | 177.6 (163.2,192) | <0.001 | 161.7 (159.1,164.2) | 169.1 (165,173.3)a | 170 (163,177) | 173.6 (166.4,180.8)a | <0.001 |
| TG (mg/dL) | 88.0 (82.4, 94.1) | 94.3 (88.5, 100.4) | 109.0 (102.3,116)ab | 123.4 (103,147.8)a | <0.001 | 91.6 (87.3,96.1) | 100.9 (92.7,109.7) | 109.6 (97.4,123.3)a | 112.4 (102.3,123.6)a | <0.001 |
| HDL-C (mg/dL) | 49.4 (48.1,50.6) | 48.6 (47.6,49.6) | 46.0 (44.6,47.4)ab | 44.9 (42.0, 47.7)ab | <0.001 | 48.9 (48.1,49.8) | 47.4 (45.7,49.1) | 44.6 (41.9,47.3)a | 46.4 (44.7,48)a | 0.002 |
| LDL-C (mg/dL) | 90.5 (87.7,93.3) | 93.6 (90.9,96.4) | 100.7 (97.1,104.3)ab | 105.3(95.1,115.5)a | <0.001 | 91.8 (89.6,94) | 98.3 (94.6,101.9)a | 100.8 (94.6,107)a | 101.9 (95.8,108.1)a | <0.001 |
| SBP (mmHg) | 109.5 (108.5,110.6) | 111.2 (110.1,112.2) | 112.3 (111.2,113.4)a | 117.1(113.1,121)ab | <0.001 | 110.5(109.7,111.3) | 111.7(110.2,113.2) | 111.7(109.5,113.8) | 113.6(111.8,115.5)a | 0.016 |
| DBP (mmHg) | 67.5 (66.5,68.5) | 68.0 (67.0, 69.0) | 68.1 (66.9,69.2) | 71.9 (68.7,75.1) | 0.081 | 67.9 (67.2,68.7) | 67.7 (66.4,69.1) | 68.5 (66.7,70.3) | 68.4 (66.8,70) | 0.859 |
| Glucose (mg/dL) | 91.6 (89.4,93.8) | 91.3 (89.9,92.6) | 92.6 (90.6,94.6) | 94.8 (91.2,98.3) | 0.251 | 92.2 (90.4,94) | 90.4 (89.2,91.5) | 92.2 (90.7,93.7) | 93 (91.1,94.8) | 0.068 |
| HbA1c | 5.5 (5.5,5.7) | 5.5 (5.4, 5.5) | 5.7 (5.5, 5.8) | 5.6 (5.5, 5.8) | 0.036 | 5.6 (5.4, 5.7) | 5.5(5.4, 5.6) | 5.6 (5.5, 5.7) | 5.6 (5.5, 5.7) | 0.083 |
Adjusted for age and sex
Data are presented as Geometric mean [95% confidence interval (CI)]
Abbreviations: SDS, standard deviation score; BMI, body mass index; TC, Total cholesterol; TG, Triglyceride; HDL-C, High density lipoprotein cholesterol; LDL-C, Low density lipoprotein cholesterol; SBP, Systolic blood pressure; DBP, diastolic blood pressure; Overweight (85th ≤BMI<95th percentile), Obesity (95th ≤BMI<120% of 95th BMI), Severe obesity (≥120% of 95th BMI or BMI 35, whichever was lower), Extreme severe obesity (≥140% of 95th BMI or BMI 40, whichever was lower); Normal waist obese (Waist Circumference, WC<90th percentile), Abdominal obesity I (90th ≤ WC<95th), abdominal obesity II (95th ≤ WC<97th), abdominal obesity III (97th ≤ WC). a, b, c: P-value <0.05 compared to overweight, obesity and severe obesity, respectively, by bonferroni corrections.
(4). Incidence of metabolic risk factors according to increasing levels of obese or central obese category.
With increasing levels of obese category, the incidence of abnormal metabolic risk factors including TC (8.2%, 8.3%, 16.2%, 20.6%, P < 0.007), HDL-C (20.2%, 18.5%, 34.4%, 43.6%, P < 0.001), LDL-C (5.2%, 6.4%, 12.8%, 17.7%, P = 0.004), TG (15.3%, 16.7%, 26.5%, 30.9%, P < 0.003), HbA1c (12.8%, 13.5%, 21.9%, 42.2%, P = 0.006), and SBP (3.5%, 6.4%, 8.1%, 19.5%, P = 0.003) showed significant differences. Among the four study groups, there was no significant difference in the prevalence of abnormal glucose or DBP (Figure 1).
Figure 1.
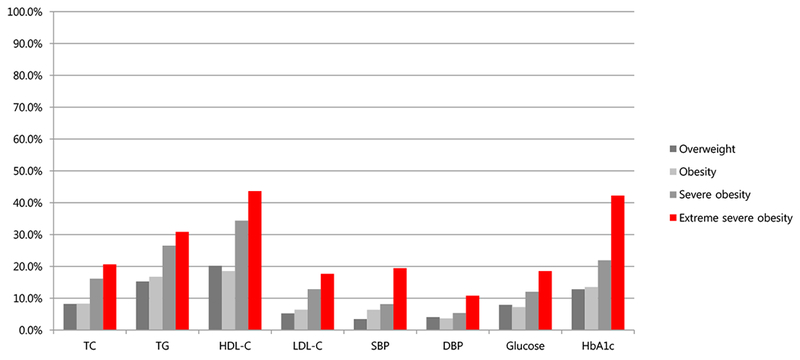
Incidence of metabolic risk factors such as TC ≥ 200 mg/dL (8.2%, 8.3%, 16.2%, 20.6%, P<0.007), HDL-C<40 mg/dL or <50 mg/dL in girls older than 16 years-old (20.2%, 18.5%, 34.4%, 43.6%, P<0.0001), LDL-C ≥130 mg/dL (5.2%, 6.4%, 12.8%, 17.7%, P<0.004), TG ≥150 mg/dL (15.3%, 16.7%, 26.5%, 30.9%, P<0.003), glucose ≥ 100 mg/ dL (7.9%, 7.2%, 12.0%, 18.5%, P= NS), HbA1c ≥ 5.8% (12.8%, 13.5%, 21.9%, 42.2%, P<0.006), SBP ≥ 130 mg/dL (3.5%, 6.4%, 8.1%, 19.5%, P<0.003), DBP ≥ 85 mg/dL (4.1%, 3.6%, 5.4%, 10.8%, P= NS) according to obese category such as overweight (85th ≤BMI<95th percentile); obesity (95th ≤BMI<120% of 95th BMI); severe obesity (≥120% of 95th BMI or BMI 35, whichever was lower); marked severe obesity (≥140% of 95th BMI or BMI 40, whichever was lower) in 10-18 year-old obese adolescents.
With increasing levels of central obese category, the incidence of abnormal metabolic risk factors including TC (8.2%, 14.1%, 12.2%, 18.9%, P = 0.011), HDL-C (20.2%, 26.2%, 37.9%, 35.7%, P < 0.001), LDL-C (6.2%, 9.2%, 12.4%, 14.8%, P = 0.023), TG (16.1%, 21.2%, 25.8%, 29.8%, P < 0.004), glucose (7.7%, 7.3%, 11.7%, 17.4%, P = 0.009), and SBP (5.1%, 7.1%, 3.0%, 13.9%, P = 0.002) showed significant differences. Among the four groups, there was no significant difference in the incidence of abnormal HbA1C or DBP (Figure 2).
Figure 2.
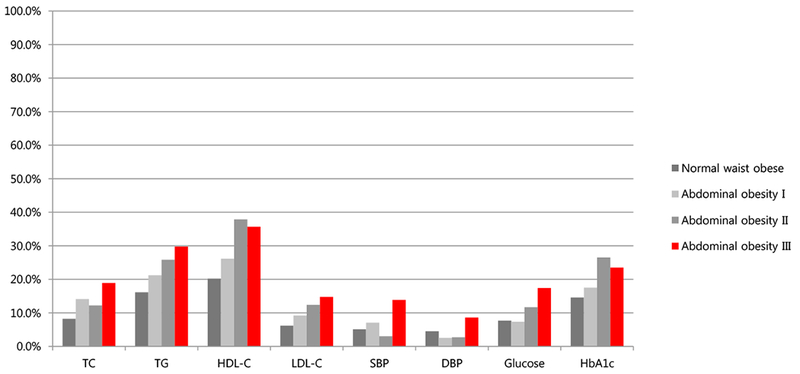
Incidence of metabolic risk factors such as TC ≥ 200 mg/dL (8.2%, 14.1%, 12.2%, 18.9%, P<0.01), HDL-C<40 mg/dL or <50 mg/dL in girls older than 16 years-old (20.2%, 26.2%, 37.9%, 35.7%, P<0.0007), LDL-C ≥ 130 mg/dL (6.2%, 9.2%, 12.4%, 14.8%, P<0.02), TG ≥ 150 mg/dL (16.1%, 21.2%, 25.8%, 29.8%, P<0.004), glucose ≥ 100 mg/dL (7.7%, 7.3%, 11.7%, 17.4%, P<0.009), HbA1c ≥ 5.8% (14.6%, 17.5%, 26.5%, 23.5%, P=NS), SBP ≥ 130 mg/dL (5.1%, 7.1%, 3.0%, 13.9%, P<0.002), DBP ≥ 85 mg/dL (4.5%, 2.6%, 2.7%, 8.6%, P=NS) according to central obese category such as normal waist obese (WC<90th percentile), abdominal obesity I (90th ≤ WC<95th), abdominal obesity II (95th ≤ WC<97th); abdominal obesity III (97th ≤ WC)) in 10-18 year-old obese adolescents.
(5). Multivariate logistic regression analyses of having metabolic risk factors according to increasing levels of obese or central obese category.
With increasing levels of obese category, the odds ratio of having abnormal metabolic risk factors including TC (P = 0.003), TG (P < 0.001), HDL-C (P < 0.001), LDL-C (P < 0.001), SBP (P = 0.004), Glucose (P = 0.023), HbA1C (P = 0.002) significantly increased after adjusting for age, sex and income. With increasing levels of central obese category, the odds ratio of having abnormal metabolic risk factors including TC (P < 0.001), TG (P < 0.001), HDL-C (P = 0.002), LDL-C (P = 0.002), SBP (P = 0.015), Glucose (P = 0.002), HbA1C (P = 0.027) significantly increased after adjusting for age, sex and income (Table 3).
Table 3.
Multivariate logistic regression analyses of having metabolic risk factors according to increasing levels of obese or central obese category.
| Overweight | Obesity | Severe obesity | Extreme severe obesity | P-value | Normal waist obese | Abdominal obesity I | Abdominal obesity II | Abdominal obesity III | P-value | |
|---|---|---|---|---|---|---|---|---|---|---|
| OR(95% CI) | OR(95% CI) | OR(95% CI) | OR(95% CI) | OR(95% CI) | OR(95% CI) | |||||
| TC | 1 | 1.0 (0.5-1.8) | 2.0 (1.2-3.6) | 2.8 (1.0-8.2) | 0.003 | 1 | 1.7 (1.0-3.0) | 1.6 (0.7-4.1) | 2.6 (1.5-4.6) | <0.001 |
| TG | 1 | 1.1 (0.7-1.8) | 2.1 (1.3-3.2) | 2.6 (1.1-6.2) | <0.001 | 1 | 1.4 (0.9-2.3) | 1.8 (1.0-3.2) | 2.2 (1.4-3.6) | <0.001 |
| HDL-C | 1 | 1.0 (0.6-1.5) | 2.0 (1.2-3.1) | 2.4 (1.1-5.3) | <0.001 | 1 | 1.5 (0.9-2.4) | 2.5 (1.5-4.4) | 1.8 (1.1-2.8) | 0.002 |
| LDL-C | 1 | 1.2 (0.6-2.6) | 2.6 (1.3-5.0) | 3.9 (1.3-12.2) | <0.001 | 1 | 1.4 (0.7-2.7) | 2.2 (0.9-5.5) | 2.7 (1.4-5.3) | 0.002 |
| SBP | 1 | 2.2 (1.0-5.2) | 2.4 (1.1-5.2) | 6.1 (1.9-20.0) | 0.004 | 1 | 2.0 (0.8-4.7) | 0.7 (0.2-3.2) | 2.7 (1.3-5.5) | 0.015 |
| DBP | 1 | 0.9 (0.4-2.4) | 1.2 (0.5-2.9) | 2.2 (0.6-7.9) | 0.359 | 1 | 0.8 (0.2-2.9) | 0.8 (0.3-2.5) | 1.6 (0.8-3.6) | 0.281 |
| Glucose | 1 | 0.9 (0.5-1.7) | 1.7(1.0-3.0) | 3.1(0.9-10.3) | 0.023 | 1 | 0.9 (0.5-1.6) | 1.6 (0.7-3.9) | 2.8 (1.5-5.3) | 0.002 |
| HbA1c | 1 | 0.9(0.5-1.9) | 2.0 (1.0-4.0) | 6.3 (1.9-21.1) | 0.002 | 1 | 1.3 (0.7-2.5) | 2.0 (0.8-4.9) | 2.1(1.0-4.4) | 0.027 |
Adjusted for age, sex and income
Abbreviations: TC (Total cholesterol ≥ 200 mg/dL), HDL-C (High density lipoprotein cholesterol <40 mg/dL or <50 mg/dL in girls older than 16 years-old), LDL-C (Low density lipoprotein cholesterol ≥130 mg/dL), TG(Triglyceride ≥150 mg/dL), glucose (≥100 mg/dL), HbA1c (≥5.8%), SBP (Systolic blood pressure ≥ 130 mg/dL), DBP(Diastolic blood pressure ≥85 mg/dL) of according to obese category, overweight (85th ≤BMI<95th percentile); obesity (95th ≤BMI<120% of 95th BMI); severe obesity (≥120% of 95th BMI or BMI 35, whichever was lower); Extreme severe obesity (≥140% of 95th BMI or BMI 40, whichever was lower) and central obese category, normal waist obese (Waist Circumference, WC<90th percentile), abdominal obesity I (90th ≤ WC<95th), abdominal obesity II (95th ≤ WC<97th); abdominal obesity III (97th ≤ WC).
4. Discussion
In this representative sample of data, the prevalence of overweight, obesity, severe obesity and extreme severe obesity were 5.6%, 6.2%, 5.9% and 0.9% in 10-18-year-old Korean adolescents. In the United States, the prevalence of overweight, obesity, severe obesity and extreme severe obesity were 14.8%, 11.3%, 3.6% and 1.5% in 2-19-year-old adolescents [2]. The prevalence of the adolescents with obesity in Korea is lower than that of in the United States [18]. In spite of lower prevalence of obesity in adolescents, the prevalence of severe degree of obesity in Korean adolescents is similar to that in the United States. Among Korean adolescents with obesity, the proportions of overweight, obesity, severe obesity, and extreme severe obesity were 30.0%, 33.4%, 32.0% and 4.7%. Among 8579 American 2-19-year-old obese subjects, the proportions of overweight, obese, severe obesity, and extreme severe obesity were 46.9%, 36.4%, 11.9%, and 4.8%, respectively [15]. In Korean adolescents, severe degree of obesity account for high proportion of total obese subjects compared to that of in the United States.
Children with obesity have been shown to be more likely to have obesity related morbidity [19,20]. Compared to mild degree of adiposity and normal weight, severe degree of pediatric obesity is associated with having higher metabolic risk factors [12]. In severe form obesity, stronger associations with abnormal metabolic risk factors have been reported compared to those with simple obesity [15]. Severe degree of pediatric obesity, compared with milder degree of adiposity and normal weight, is associated with higher levels of cardiorespiratory deconditioning, endothelial dysfunction, and clustering of cardiometabolic risk factors [1,8,12,21,22]. In the present study, we also found increased prevalence of abnormal metabolic risk factors including TC, HDL-C, LDL-C, TG, HbA1c, and SBP with increasing levels of obese category in Korean adolescents after adjusting for age and sex. The higher incidence of abnormal metabolic risk factors in more severe obesity in comparison with less severe form obesity suggests that a more concise classification of obesity in adolescents is necessary.
Visceral fat is a major contributing factor in insulin resistance and risk of type 2 diabetes and coronary heart disease [23–25]. Visceral adipose tissue carries a greater risk for cardiovascular disorders than does subcutaneous adipose tissue [26]. We previously reported that subjects classified as centrally obese normal weight have higher insulin resistance than obese subjects without central obesity, particularly in Korean male adolescents [27]. In the present study, 10-18-year-old adolescents with obesity were further classified by increasing levels of central obese category to assess central obesity. We also found differences increased prevalence of abnormal metabolic syndrome components including TC, HDL-C, LDL-C, TG, HbA1C, and SBP with increasing levels of central obese category in adolescents with obesity after adjusting for age and sex. Our data suggest that early recognition of severe form obesity and complications associated with obesity are very important.
Unfortunately, many of the treatment approaches commonly used with some success in overweight and obese youth are less effective in those with severe obesity [28,29]. Diet, exercise, and behavioral modification are recommended as initial treatments for severe obesity, resulting in short-term weight loss, which, when combined with pharmacotherapy, is associated with a 5% to 10% reduction in weight [30]. However, anti-obesity pharmacological agents have substantial adverse effects, and discontinuation often results in weight regain [30]. In adolescents with severe obesity and serious comorbidities, bariatric surgery has been considered for weight loss because of the poor success of nonsurgical treatment in achieving and maintaining weight loss [31]. However, this procedure has major potential complications, including leakage, pneumonia, pulmonary embolism, band slippage, and band erosion [32]. Given the lack of successful treatment options and complications associated with treatment, prevention of severe pediatric obesity is the ultimate goal.
In the present study, 5.9% and 0.9% of Korean adolescents were found to be severe obesity and extreme severe obesity using data from the 2007-2014 K-NHANES. Our results show that adolescents with severe obesity have much greater metabolic risk components than adolescents with less severe degree of obesity. In this study, the subjects were aged 10–18 years and not homogenous in terms of the degree of sexual maturation. Data from K-NHANES didn’t include information about puberty. For adjusting heterogeneity in terms of the degree of maturation, we classified central obesity and obesity as those in according to the age and sex specific BMI or WC percentile. In logistic regression, sex and age were adjusted as confounding variables. These findings in this study suggests that there are significant adverse effects of severe obesity on each component of metabolic syndrome, emphasizing the deleterious effects of severe obesity on the health of adolescents. The present study point to the need for detailed strategies for the prevention and management of severe obesity in adolescents.
Acknowledgement:
Funding: None.
Footnotes
Abbreviations: None.
Conflict of interest: The authors declare no conflict of interest.
Financial Disclosure: The authors have no financial relationships relevant to this article to disclose.
Reference
- [1].Kelly AS, Barlow SE, Rao G, Inge TH, Hayman LL, Steinberger J, et al. Severe obesity in children and adolescents: identification, associated health risks, and treatment approaches: a scientific statement from the American Heart Association. Circulation 2013;128:1689–712. [DOI] [PubMed] [Google Scholar]
- [2].Skinner AC, Skelton JA. Prevalence and trends in obesity and severe obesity among children in the United States, 1999-2012. JAMA Pediatr 2014;168:561–6. [DOI] [PubMed] [Google Scholar]
- [3].Finkelstein EA, Khavjou OA, Thompson H, Trogdon JG, Pan L, Sherry B, et al. Obesity and severe obesity forecasts through 2030. Am J Prev Med 2012;42:563–70. [DOI] [PubMed] [Google Scholar]
- [4].Calle EE, Thun MJ, Petrelli JM, Rodriguez C, Heath CW Jr. Body-mass index and mortality in a prospective cohort of U.S. adults. N Engl J Med 1999;341:1097–105. [DOI] [PubMed] [Google Scholar]
- [5].Whitaker RC, Wright JA, Pepe MS, Seidel KD, Dietz WH. Predicting obesity in young adulthood from childhood and parental obesity. N Engl J Med 1997;337:869–73. [DOI] [PubMed] [Google Scholar]
- [6].Morrison JA, Friedman LA, Gray-McGuire C. Metabolic syndrome in childhood predicts adult cardiovascular disease 25 years later: the Princeton Lipid Research Clinics Follow-up Study. Pediatrics 2007;120:340–5. [DOI] [PubMed] [Google Scholar]
- [7].Freedman DS, Shear CL, Burke GL, Srinivasan SR, Webber LS, Harsha DW, et al. Persistence of juvenile-onset obesity over eight years: the Bogalusa Heart Study. Am J Public Health 1987;77:588–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Norris AL, Steinberger J, Steffen LM, Metzig AM, Schwarzenberg SJ, Kelly AS. Circulating oxidized LDL and inflammation in extreme pediatric obesity. Obesity (Silver Spring) 2011;19:1415–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Freedman DS, Mei Z, Srinivasan SR, Berenson GS, Dietz WH. Cardiovascular risk factors and excess adiposity among overweight children and adolescents: the Bogalusa Heart Study. J Pediatr 2007;150:12–7. e2. [DOI] [PubMed] [Google Scholar]
- [10].Srinivasan SR, Bao W, Wattigney WA, Berenson GS. Adolescent overweight is associated with adult overweight and related multiple cardiovascular risk factors: the Bogalusa Heart Study. Metabolism 1996;45:235–40. [DOI] [PubMed] [Google Scholar]
- [11].Must A, Jacques PF, Dallal GE, Bajema CJ, Dietz WH. Long-term morbidity and mortality of overweight adolescents. A follow-up of the Harvard Growth Study of 1922 to 1935. N Engl J Med 1992;327:1350–5. [DOI] [PubMed] [Google Scholar]
- [12].Weiss R, Dziura J, Burgert TS, Tamborlane WV, Taksali SE, Yeckel CW, et al. Obesity and the metabolic syndrome in children and adolescents. N Engl J Med 2004;350:2362–74. [DOI] [PubMed] [Google Scholar]
- [13].Centers for Disease Control & Prevention. Korean national health and nutrition examination survey [cited 2013 July 1]. Available from: http://knhanes.cdc.go.kr/
- [14].Moon JS, Lee SY, Nam CM, Choi JM, Choe BK, Seo JW, et al. 2007 Korean National Growth Charts: review of developmental process and an outlook. Korean J Pediatr 2008;51:1–25. [Google Scholar]
- [15].Skinner AC, Perrin EM, Moss LA, Skelton JA. Cardiometabolic Risks and Severity of Obesity in Children and Young Adults. N Engl J Med 2015;373:1307–17. [DOI] [PubMed] [Google Scholar]
- [16].Cook S, Weitzman M, Auinger P, Nguyen M, Dietz WH. Prevalence of a metabolic syndrome phenotype in adolescents: findings from the third National Health and Nutrition Examination Survey, 1988-1994. Arch Pediatr Adolesc Med 2003;157:821–7. [DOI] [PubMed] [Google Scholar]
- [17].Zimmet P, Alberti KG, Kaufman F, Tajima N, Silink M, Arslanian S, et al. The metabolic syndrome in children and adolescents - an IDF consensus report. Pediatr Diabetes 2007;8:299–306. [DOI] [PubMed] [Google Scholar]
- [18].Park J, Hilmers DC, Mendoza JA, Stuff JE, Liu Y, Nicklas TA. Prevalence of metabolic syndrome and obesity in adolescents aged 12 to 19 years: comparison between the United States and Korea. J Korean Med Sci 2010;25:75–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Jeon YJ, Jung IA, Kim SH, Cho WK, Jeong SH, Cho KS, et al. Serum ferritin level is higher in male adolescents with obesity: results from the Korean National Health and Nutrition Examination Survey 2010. Ann Pediatr Endocrinol Metab 2013;18:141–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Barlow SE. Expert committee recommendations regarding the prevention, assessment, and treatment of child and adolescent overweight and obesity: summary report. Pediatrics 2007;120 Suppl 4:S164–92. [DOI] [PubMed] [Google Scholar]
- [21].Gidding SS, Nehgme R, Heise C, Muscar C, Linton A, Hassink S. Severe obesity associated with cardiovascular deconditioning, high prevalence of cardiovascular risk factors, diabetes mellitus/hyperinsulinemia, and respiratory compromise. J Pediatr 2004;144:766–9. [DOI] [PubMed] [Google Scholar]
- [22].Tounian P, Aggoun Y, Dubern B, Varille V, Guy-Grand B, Sidi D, et al. Presence of increased stiffness of the common carotid artery and endothelial dysfunction in severely obese children: a prospective study. Lancet 2001;358:1400–4. [DOI] [PubMed] [Google Scholar]
- [23].Patel P, Abate N. Body fat distribution and insulin resistance. Nutrients 2013;5:2019–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].De Lorenzo A, Del Gobbo V, Premrov MG, Bigioni M, Galvano F, Di Renzo L. Normal-weight obese syndrome: early inflammation? Am J Clin Nutr 2007;85:40–5. [DOI] [PubMed] [Google Scholar]
- [25].Vanhala M Childhood weight and metabolic syndrome in adults. Ann Med 1999;31:236–9. [DOI] [PubMed] [Google Scholar]
- [26].Manolopoulos KN, Karpe F, Frayn KN. Gluteofemoral body fat as a determinant of metabolic health. Int J Obes (Lond) 2010;34:949–59. [DOI] [PubMed] [Google Scholar]
- [27].Cho WK, Kim H, Lee HY, Han KD, Jeon YJ, Jung IA, et al. Insulin Resistance of Normal Weight Central Obese Adolescents in Korea Stratified by Waist to Height Ratio: Results from the Korea National Health and Nutrition Examination Surveys 2008–2010. Int J Endocrinol 2015;2015:158758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Danielsson P, Kowalski J, Ekblom O, Marcus C. Response of severely obese children and adolescents to behavioral treatment. Arch Pediatr Adolesc Med 2012;166:1103–8. [DOI] [PubMed] [Google Scholar]
- [29].Savoye M, Nowicka P, Shaw M, Yu S, Dziura J, Chavent G, et al. Long-term results of an obesity program in an ethnically diverse pediatric population. Pediatrics 2011;127:402–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Bray GA. Drug treatment of obesity. Rev Endocr Metab Disord 2001;2:403–18. [DOI] [PubMed] [Google Scholar]
- [31].Styne DM, Arslanian SA, Connor EL, Farooqi IS, Murad MH, Silverstein JH, et al. Pediatric Obesity-Assessment, Treatment, and Prevention: An Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab 2017;102:709–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Tanner BD, Allen JW. Complications of bariatric surgery: implications for the covering physician. Am Surg 2009;75:103–12. [DOI] [PubMed] [Google Scholar]


