Abstract
MicroRNAs (miRNAs) are small non-coding RNAs that typically inhibit the translation and stability of messenger RNAs (mRNAs). They are ~22 nucleotides long and control both physiological and pathological processes. Altered expression of miRNAs is often associated with human diseases. Thus, miRNAs have become important therapeutic targets, and some clinical trials investigating the effect of miRNA-based therapeutics in different types of diseases have already been conducted. The tumor microenvironment (TME) comprises cells such as infiltrated immune cells, cancer-associated endothelial cells (CAEs) and cancer-associated fibroblasts (CAFs), and all the components participate in the complicated crosstalk with tumor cells to affect tumor progression. Altered miRNAs expression in both these stromal and tumor cells could drive tumorigenesis. Thus, in this review, we discuss how aberrantly expressed miRNAs influence tumor progression; summarize the crosstalk between infiltrated immune cells, CAEs, CAFs, and tumor cells through miRNAs, and clarify the important roles of miRNAs in the tumor microenvironment, which may facilitate the clinical application of miRNA-based therapies.
Keywords: microRNAs, immune cells, cancer-associated endothelial cells, cancer-associated fibroblasts, tumor cells, crosstalk, tumor microenvironment
Introduction
MicroRNAs (miRNAs) are functional single-strand RNAs, ~22 nucleotides long, which are transcribed in the nucleus first by RNA polymerase II, called pri-miRNAs. After they are cleaved by Drosha and DGCR8, pri-miRNAs are transferred to pre-miRNAs, and then with the aid of exportin 5, pre-miRNAs were transported to the cytoplasm and processed by Dicer, leading to the formation of double-stranded microRNA, and then the dimeric RNAs are unwound by Argonaute proteins and incorporated into RNA-induced silencing complex (RISC). RISC directly binds to the 3′ untranslated region (UTR) of targeted mRNAs to degrade or repress translation (1).
The first miRNA was identified in Caenorhabditis elegans by Lee et al. (2), who found that a short RNA product encoded by lin-4 could partially complement the 3′ UTR of lin-14 mRNA, reduce the amount of lin-14 protein, and regulate the development of C. elegans. Following the discovery of the second miRNA, let-7, which is conserved in many organisms, the roles of miRNAs in non-nematode species became apparent (3). To date, miRNAs have been found to participate in many physiological processes such as cell cycle regulation, proliferation, apoptosis, and neurogenesis (4). miRNAs regulate up to 60% of protein-coding genes (5), and the deregulation of miRNAs is associated with various human diseases, including cancer, autoimmune diseases, cardiovascular and neurological disorders (6).
In tumor cells, aberrantly expressed miRNAs exert tumor-suppressive or oncogenic functions by regulating the expression of mRNAs in different signaling pathways, thereby affecting tumor progression (7). The miR-15 and miR-16 were the first miRNAs linked to cancer. In 2002, Calin et al. found they were downregulated in most patients with chronic lymphocytic leukemia (CLL) (8). Furthermore, miR-155, miR-21, and miR-210 expression levels are elevated in the sera of patients with diffuse large B-cell lymphoma (DLBCL) (9). These observations suggested the use of miRNAs as diagnostic and prognostic biomarkers. miRNAs are also regarded as therapeutic targets in human cancer, and several related clinical trials have been conducted (10).
The tumor microenvironment (TME) is very complex and in addition to tumor cells, comprises several infiltrated cell types such as infiltrated immune cells, cancer-associated endothelial cells (CAEs), and cancer-associated fibroblasts (CAFs). These cells significantly contribute to tumor progression, and deregulated miRNAs expression in these cells might determine the fate of the tumor. In this review, we summarize the role of miRNAs in the crosstalk between these cells and tumor cells, to enhance our understanding of the significance of miRNAs in the TME and lay a foundation for miRNA-based therapies in cancer treatment.
miRNAs play a role in the modulation of the TME
miRNAs as modulators between infiltrated immune cells and tumor cells
Different types of infiltrated immune cells are involved in the TME and contribute to the regulation of the fine balance between anti- and pro-tumor signals. Among them, lymphocytes (especially T cells), natural killer cells (NKs), and dendritic cells (DCs) are crucial for tumor suppression, while regulatory T cells (Tregs), myeloid-derived suppressor cells (MDSCs), and tumor-associated macrophages (TAMs) are considered to play immunosuppressive roles. As multifaceted regulators, miRNAs influence the differentiation and function of the immune cells mentioned above. The miR-155, for example, has been reported to be widely expressed in immune cells. In T cells, it was initially found to regulate the Th cell lineage decision, because miR-155-deficient mice display a bias toward Th2 differentiation (11). Tregs proliferation and homeostasis can also be modulated by miR-155 (12). In NKs, miR-155 was found to promote NK expansion and functional activation and enhance the production of IFN-γ (13, 14). miR-155 deficiency in DCs impairs their maturation, migration ability, cytokine production, and ability to activate T cells (15). In MDSCs, miR-155 is involved in the expansion of MDSCs and is required to facilitate tumor growth (16). In TAMs, the downregulation of miR-155 increases the production of IL-10, enhancing its immunosuppressive function (17). The roles of miRNAs in immune and cancer cells have been thoroughly reviewed elsewhere (7, 18), while the miRNA-mediated crosstalk between tumor-infiltrated immune cells and tumor cells in the TME remains to be elucidated. Hence, in this section, we will discuss several representative and well-studied miRNAs that act as critical modulators of the immune response in the TME.
miRNAs as modulators between T cells and tumor cells
T lymphocyte-mediated immune responses are critical for tumor immunity. After activation, CD4+ T cells differentiate into different types of T helper cells (Th1, Th2, Th17, and Tfh) and Tregs. miRNAs have been increasingly recognized as important modulators of Th cells and Tregs fate decisions and effector functions (19). Th2 and Th17 cells mainly mediate responses against helminths, bacteria, and fungi, and although they are necessary for effective T cell-dependent antibody responses, they do not participate directly in tumor immunity. Thus, the miRNA-mediated crosstalk with these cells will not be discussed here.
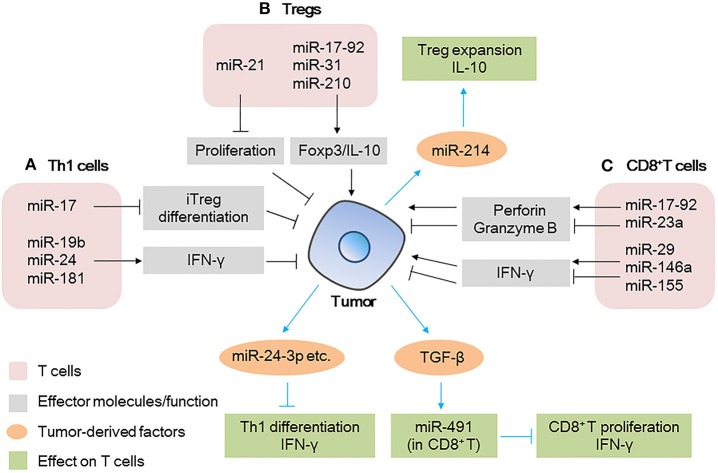
Th1 cells are the main CD4+ T cell population involved in the response against tumors. The production of IFN-γ is a hallmark of Th1 cells function in the TME. miRNAs such as the miR-17-92 cluster, miR-24, and miR-181 participate in the production of IFN-γ (20, 21) (Figure 1A). The miR-17-92 cluster, for example, plays a key role in controlling Th1 cells responses through multiple coordinated processes. Deficiency of miR-17-92 cluster in CD4+ T cells significantly impairs the Th1 cells response to B16 tumor cells, including a decrease in Th1 cells number and IFN-γ production and impairment in the ability of CD4+ T cells to help CD8+ T cells. Two members of the miR-17-92 cluster, miR-19b and miR-17, cooperate and inhibit tumor progression. Specifically, miR-19b directly targets PTEN, enhances the activity of the PI3K/AKT signaling pathway, promotes the proliferation of Th1 cells and the production of IFN-γ, and inhibits the differentiation of iTreg. miR-17 targets CREB1 and TGFβ-II and inhibits the differentiation of iTreg (20). These data suggest that the inhibition of the miR-17-92 cluster might subvert the immune response against tumors.
Figure 1.
MicroRNAs (miRNAs) act as modulators between T cells and tumor cells (A) miRNAs expressed in Th1 cells modulate tumor progression by inducing iTreg differentiation or secreting IFN-γ; tumor-derived miRNAs affect the differentiation/IFN-γ production by Th1 cells. (B) miRNAs expressed in Tregs modulate tumor progression by regulating transcription factor expression or cytokine production; tumor-derived miRNAs affect the expansion/cytokine production in Tregs. (C) miRNAs expressed in CD8+ T cells modulate tumor progression by regulating effector molecule (IFN-γ and perforin/granzyme B) production; tumor-derived factors affect miRNAs expression in CD8+ T cells, further affect the proliferation/IFN-γ production by CD8+ T cells.
miRNAs expressed in tumor cells affect the function of Th1 cells (Figure 1A). For example, miRNAs in tumor-derived microvesicles (MVs)/exosomes such as miR-24-3p, miR-891a, miR-106a-5p, miR-20a-5p, and miR-1908, have been found to impair T cell function by inhibiting Th1 and Th17 differentiation; downregulating the MAPK pathway; affecting the secretion of cytokines such as IL-1β, IL-6, IL-10, IFN-γ, IL-2, and IL-17, and reducing the antitumor effect (22).
Tregs are important in maintaining immunosuppression. Many miRNAs such as miR-21, miR-126, miR-142-3p, miR-146, and miR-155 have been reported to regulate the differentiation, maintenance, and function of Tregs (12, 23–26). Regarding the function of Tregs in the TME, miR-21 has been found to be highly expressed in CCR6+ Tregs in tumor tissues from a murine breast cancer model. Silencing of miR-21 altered the enrichment of CCR6+ Tregs in the tumor mass and enhanced the antitumor effect of CD8+ T cells. Mechanistic evidence has shown that miR-21 targets PTEN, alters the activation of the AKT pathway, and reduces the proliferation of CCR6+ Tregs, abrogating their immunosuppressive capacity (26). Other miRNAs, such as miR-31 (27), miR-210 (28), and the miR-17-92 cluster (29) have been reported to regulate the expression of Foxp3 and IL-10 and affect the immunosuppressive function of Tregs (Figure 1B), but their roles in the TME remain unclear. This indicates that the targeting of specific miRNAs in Tregs is promising in the development of therapeutic strategies against tumors.
Tumor-derived factors such as miRNAs could also be taken up by Tregs, further affecting the immune response (Figure 1B). The miR-214 secreted by various human cancers and mouse tumor models is delivered to recipient Tregs by MVs, efficiently downregulates PTEN, promotes Tregs expansion, and enhances the production of IL-10 in vitro (30). Specifically, the authors found that in a lung carcinoma model in nude mice, miR-214 increased the secretion of IL-10 by Tregs and promoted tumor growth. However, when anti-miR-214 antisense oligonucleotides (ASOs) were delivered to mice implanted with tumors, the expansion of Tregs was blocked and tumor growth was inhibited (Figure 1B). This revealed a novel mechanism through which cancer cells actively manipulate the immune response by promoting Tregs expansion (30).
The antitumor effect of CD8+ T cells in the TME can be evaluated by the cytokines (mainly IFN-γ) and cytotoxic molecules (mainly perforin and granzyme B) they produce. The process can also be regulated by miRNAs. Several research groups have identified unique miRNAs that regulate CD8+ T cell production of IFN-γ, such as miR-29 (31), miR-146a, and miR-155 (32) (Figure 1C). For example, in a mouse melanoma model, researchers found restricted tumor growth in miR-146a-deficient mice and enhanced tumor activity in miR-155-deficient mice. miR-155 seemed to play a more dominant role than that of miR-146a, because in mice lacking both miR-146a and miR-155, CD8+ T cells show defects in IFN-γ expression and antitumor immunity, a phenotype similar to that observed in CD8+ T cells of miR-155-deficient mice (32). Similarly, another group found that when miR-155 was overexpressed in CD8+ T cells, the survival of tumor-challenged mice was prolonged significantly (33).
miRNAs also mediate CD8+ T cells effector responses other than IFN-γ production, such as the secretion of perforin and granzyme B (Figure 1C). For example, the miR-17-92 cluster (34) and miR-23a (35) have been reported to regulate the expression of these cytotoxic molecules in CD8+ T cells. miR-17-92-deficient CD8+ T cells failed to upregulate T-bet and Eomes through an unknown mechanism, which ultimately decreased the production of perforin and granzyme B (34). On the other hand, miR-23a has been found to be upregulated in tumor-infiltrating CD8+ T cells of patients with lung cancer, where it acts as a repressor of the transcription factor BLIMP-1, which promotes CD8+ T cell cytotoxicity. The inhibition of miR-23a enhances granzyme B expression in human CTLs and robustly hinders tumor progression in mice with established melanoma tumors. These data indicate that by modulating different signaling pathways, the miR-17-92 cluster and miR-23a play opposite roles in regulating the function of CD8+ T cells, suggesting the multifaceted function of miRNAs in the TME.
Moreover, tumor-derived factors influence the miRNAs expression of CD8+ T cells (Figure 1C), tumor-derived TGF-β induce the expression of miR-491 in CD8+ T cells. High expression level of miR-491 decreased cell proliferation, increased apoptosis, and decreased IFN-γ production by CD8+ T cells. These effects are exerted through the targeting of Bcl-xL, CDK4, and TCF-1, thereby enhancing tumor immune escape (36).
Immune checkpoint molecules expressed on T cells surfaces such as PD-1, CTLA-4, TIM-3, BTLA, and LAG3 are particularly appealing for cancer therapy. Evidence has shown that the expression of these molecules could be regulated by miRNAs, and they influence tumor progression by different mechanisms (37–42), and representative miRNAs involved in these processes are indicated in Table 1. In addition, checkpoint molecules such as PD-L1 expressed on tumor cells can also be regulated by miRNAs, and they affect the function of immune cells and alter the behavior of tumor cells (43–48), as summarized in Table 2.
Table 1.
Representative immune cell microRNAs (miRNAs) related to the immune response in the tumor microenvironment (TME).
| miRNAs | Host cells | Target molecules | Expression level | Immune-related roles in the TME | References |
|---|---|---|---|---|---|
| miR-15a/16 | T | PD-1, TIM-3, LAG3 | Downregulated | Enhances the activation of tumor-infiltrating CD8+T cells | (42) |
| miR-28 | T | PD-1, TIM-3, BTLA | Downregulated | Inhibits T cell exhaustion, regulates the secretion of cytokines (IL-2 and TNF-α) | (39) |
| miR-138 | T | PD-1, CTLA-4 | Downregulated | Exerts anti-glioma efficacy | (41) |
| miR-182 | NK | NKG2A NKG2D | Upregulated | Induces NK-cell cytotoxicity (perforin-1 upregulation and increase in cytolytic killing ability) | (38) |
| miR-1245 | NK | NKG2D | Downregulated | Promotes cancer immunosurveillance | (37) |
| miR-30c | NK | NKG2D CD107a, FasL | Upregulated | Enhances NK cell activation and cytotoxicity to tumor cells | (40) |
Table 2.
Representative tumor cell microRNA (miRNAs) related to immune response in tumor microenvironment (TME).
| miRNAs | Host cells | Target molecules | Expression level | Immune-related roles in the TME | References |
|---|---|---|---|---|---|
| miR-BART-2, 4, 5, 18, 22 | CRC, GC | PD-L1 | Upregulated | Suppress immune responses, enhance the secretion of TGF-β and IL-10 | (45) |
| miR-200 | NSCLC | PD-L1 | Downregulated | Reverses CD8+T cell exhaustion | (43) |
| miR-20b, 21, 130b | CRC | PD-L1 | Upregulated | Mitigate T cell activation | (44) |
| miR-424(322) | OC | PD-L1 | Downregulated | Promotes the proliferation of functional cytotoxic CD8+ T cells and inhibits MDSCs and Tregs | (47) |
| miR-34a | AML | PD-L1 | Downregulated | Reduces specific T cell apoptosis | (46) |
| miR-142-5p | PC | PD-L1 | Downregulated | Increases CD4+T cells and CD8+ T cells, decreases PD-1+ T cells, increases IFN-γ and TNF-α | (48) |
CRC, colorectal cancer; GC, gastric cancer; NSCLC, non-small cell lung cancer; OC, ovarian cancer; AML, acute myelocytic leukemia; PC, pancreatic cancer.
Collectively, these data support the notion that the widespread changes in miRNA expression in T cells affect tumorigenesis and determine the behavior of cancer. Therefore, deregulated miRNAs in T cells may be regarded as potential targets in cancer immunotherapy.
miRNAs as modulators between NKs and tumor cells
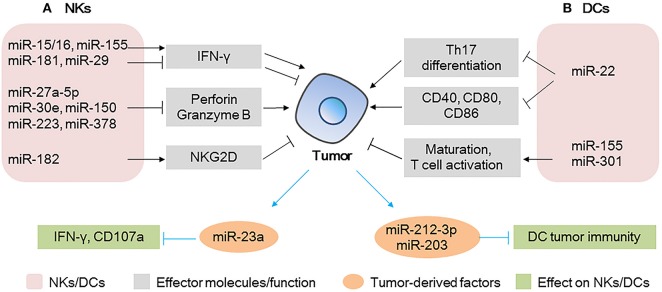
NKs can rapidly respond to the presence of tumor cells and initiate an antitumor immune response by producing effector molecules such as IFN-γ, perforin, and granzyme B (49, 50) (Figure 2A). The most extensively studied individual miRNA in NKs is miR-155, which promotes the production of IFN-γ by targeting SHIP1 in NKs, increases antitumor activity in vitro, and improves the survival of lymphoma-bearing mice in vivo (14). Other miRNAs such as miR-15/16, miR-29, and miR-181 also regulate the production of IFN-γ in NKs by different mechanisms (31, 51, 52) (Figure 2A).
Figure 2.
MicroRNAs (miRNAs) act as modulators between natural killer (NKs)/dendritic cells (DCs) and tumor cells (A) miRNAs expressed in NKs modulate tumor progression by regulating the production of effector molecules (IFN-γ and perforin/granzyme B) and the activating receptor NKG2D; tumor-derived miRNAs affect the IFN-γ production/CD107 expression in NKs. (B) miRNAs expressed in DCs modulate tumor progression by regulating Th17 differentiation, co-stimulatory molecules expression, or T cell activation; tumor-derived miRNAs can be taken up by DCs, affecting the tumor immunity of DCs.
A number of groups have reported that miRNAs such as miR-27a-5p, miR-30e, miR-150, miR-223, and miR-378 potentially regulate perforin or granzyme B production or both and impair the antitumor potential of NKs in the TME (53–56). Notably, miR-150-deficient NKs have higher perforin levels and increased NKs cytotoxicity than non-deficient NKs do. Moreover, it has been shown that injection of miR-150-deficient NKs in immune-deficient mice causes a significant reduction in tumor growth and metastasis of B16 melanoma. Thus, the therapeutic control of miR-150 in NKs could enhance NK-based immunotherapy against cancer, providing a better clinical outcome (56).
NKs activation status is also determined by the balance between activating and inhibitory receptors, such as NKG2D and NKG2A, respectively (Figure 2A). Researchers have demonstrated that the NKs of patients with hepatocellular carcinoma (HCC) have higher miR-182 levels than those of healthy subjects. In the same patients, NKG2D and NKG2A were upregulated and downregulated, respectively. Interestingly, miR-182 overexpression in isolated HCC NKs was associated with the upregulation of both receptors, increased production of perforin-1, and cytotoxicity of NKs when these cells were co-cultured with Huh-7 cells. This suggested that, upon overexpression of miR-182, the activation signals of NKG2D override the inhibitory signals of NKG2A, leading to enhanced cytotoxicity of NKs (38). Other miRNAs (40, 57) involved in the regulation of these receptors are summarized in Table 1.
Tumor-derived MVs are key mediators of the interactions between tumor and immune cells (Figure 2A). Berchem et al. (58) found that MVs derived from hypoxic tumor cells are taken up by NKs and affect the immune response. For example, miR-23a in hypoxic tumor-derived MVs operates as an immunosuppressive factor in NKs, by directly targeting IFN-γ and CD107a in NKs and attenuating NK function. TGF-β secreted by tumor cells can also be transferred to NKs by MVs, decreasing the cell surface expression of NKG2D and inhibiting NK function (58).
Thus, miRNAs expressed in NKs or secreted by tumor cells affect tumor behavior by manipulating the production of effector molecules or the expression of cell surface molecules. These findings highlight the potential role of miRNAs in evading immune surveillance and hence, in tumor progression.
miRNAs as modulators between DCs and tumor cells
DCs are central players in the induction of antitumoral immunity, providing critical signals that drive the induction of T cell responses. Notably, miRNAs expressed in DCs, such as miR-22 (59), miR-155 (60), and miR-301 (61) are associated with the antitumor activity of DCs and their immune regulation (Figure 2B). Researchers found that in the B16 mouse melanoma model, the overexpression of miR-22 in DCs leads to faster tumor development, larger tumor size, shorter survival time, and heavier tumors than miR-22 inhibitor group. Specifically, the authors showed that miR-22 decreased the production of IL-6, which in turn, inhibited the polarization of Th17 and the expression of IL-17A, finally promoting tumor growth (59). Furthermore, miR-22 inhibited p38, thereby downregulating CD40, CD80, and CD86 and raising the risk of DC dysfunction. Thus, miR-22 has a tumor-promoting effect and impairs the effectiveness of immunotherapy, suggesting that the inhibition of miR-22 could be a strategy to enhance the antitumor activity of DCs. Therefore, miR-22 inhibitors could serve as promising agents to improve the performance of existing DC-based therapeutic tumor vaccines (59).
miR-155, which plays important roles in various cell types, can also regulate the properties of DCs. Researchers have found that miR-155 induction is required for efficient DC maturation and is critical for the ability of DCs to promote antigen-specific T cell activation (60). The enhancement of the activity of miR-155 in ovarian cancer-associated DCs could transform them from immunosuppressive to highly immune stimulatory cells, capable of triggering potent antitumor responses and abrogating the progression of ovarian cancer (62).
miRNAs derived from tumor cells also affect DCs (Figure 2B). miR-212-3p, secreted from pancreatic cancer cells, can be transferred to DCs and inhibit RFXAP expression, resulting in decreased expression of MHC-II molecules and induced immune tolerance of DCs (63). Similarly, miR-203, derived from pancreatic cancer-secreted exosomes, can be transferred to DCs, leading to the downregulation of TLR4 and reducing the production of TNF-α and IL-12, which are necessary for the maturation and differentiation of DCs, respectively, and resulting in weakened cellular immunity (64). These findings suggest that exosome-mediated miRNA transfer between immune and tumor cells plays an important role in the modulation of the TME and that the associated miRNAs might be new potential targets for tumor immunotherapy.
miRNAs as modulators between MDSCs and tumor cells
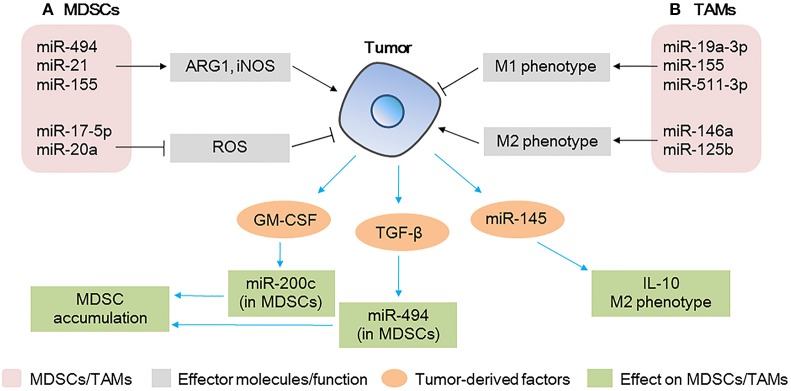
MDSCs comprise the major cell population that negatively regulates immune responses. MDSCs suppress T cells function through a number of mechanisms involving arginase 1 (ARG1), inducible NO synthase (iNOS), and reactive oxygen species (ROS) (65, 66). It has been demonstrated that some miRNAs such as miR-494, miR-155, and miR-21 are fundamental for the recruitment of MDSCs to the tumor site (Figure 3A), contributing to the modulation of their immunosuppressive function and to tumor growth by regulating the production of ARG1 and iNOS (67, 68). In contrast, miR-17-5p and miR-20a inhibit the expression of STAT3, reduce the production of ROS, and inhibit the immunosuppressive function of MDSCs (69).
Figure 3.
MicroRNAs (miRNAs) act as modulators between myeloid-derived suppressor cells (MDSCs)/tumor-associated macrophages (TAMs) and tumor cells (A) miRNAs expressed in MDSCs modulate tumor progression by regulating the expansion/immunosuppressive function of MDSCs; tumor-derived factors affect miRNA expression in MDSCs, affecting the accumulation of MDSCs at the tumor site. (B) miRNAs expressed in TAMs modulate tumor progression by regulating the polarization of TAMs; tumor-derived miRNAs affect TAM polarization and IL-10 production.
Notably, the expression of miR-494 in MDSCs downregulates the protein levels of PTEN, increases the activity of the AKT pathway, and upregulates ARG1 and iNOS, thus contributing to the accumulation of MDSCs in the tumor tissue and promoting tumor cell invasion and metastasis. Contrarily, the knockdown of miR-494 inhibits the activity of MDSCs and inhibits tumor growth and metastasis of 4T1 murine breast cancer in vivo, suggesting that miR-494 plays a key role in the expansion and maintenance of tumor-associated MDSCs (67). A different study found that the levels of miR-155 and miR-21 are increased in bone marrow and spleen MDSCs of tumor-bearing mice; the authors showed that the overexpression of miR-155 and miR-21 induced the expansion of both monocytic and granulocytic MDSCs by targeting SHIP-1 and PTEN, increased the production of ARG1 and iNOS, and enhanced immunosuppression (68). These data indicate that miRNAs play critical roles in the events governing the accumulation and function of tumor-expanded MDSCs.
Conversely, tumor-derived factors affect the function of MDSCs (Figure 3A). GM-CSF derived from tumor cells increases miR-200c levels in MDSCs and miR-200c, in turn, promotes the expansion and immunosuppressive activity of MDSCs by targeting PTEN and FOG2. Specifically, in vivo experiments have shown that miR-200c can remarkably promote tumor growth by modifying MDSCs (70). Similarly, TGF-β1, derived from breast cancer cells, increases the levels of miR-494 in MDSCs, enhances CXCR4-mediated MDSC chemotaxis, contributes to the accumulation of MDSCs in tumor tissues, and facilitates the invasion and metastasis of tumor cells (67).
MDSCs are heterogeneous populations of immature and suppressive myeloid cells that expand in nearly all diseases. In the TME, miRNAs regulate the balance between MDSCs and tumor cells and, thus, miRNAs might serve as potential therapeutic targets, both in MDSCs and tumor cells.
miRNAs as modulators between TAMs and tumor cells
TAMs are key components of the TME, and they directly affect multiple processes in tumor development. In response to microenvironmental signals, TAMs undergo M1 or M2 polarization and, therefore, exert anti-tumoral or pro-tumoral functions (71). miRNAs are involved in the polarization of TAMs (Figure 3B). The overexpression of miR-155 in TAMs re-programs anti-inflammatory, pro-tumoral M2 TAMs to pro-inflammatory, anti-tumoral M1 macrophages (71). Similarly, in breast cancer TAMs, miR-19a-3p regulates the switch from an M2-like into an M1-like phenotype by targeting Fra-1, VEGF, and STAT3, contributing to the inhibition of metastasis (72). In contrast, miR-146a promotes the expression of M2 phenotype-associated molecules and promotes 4T1 tumor growth (73). Other miRNAs expressed in TAMs such as miR-142-3p (74), miR-125b (75), and miR-511-3p (76) also play vital roles in tumor progression. These studies show that the modulation of a single miRNA in TAMs could promote the activation of a specific signaling pathway and change the fate of tumor cells in the TME.
Furthermore, a recent study has shown that miRNAs in colorectal cancer cell-derived MVs contribute to tumor development and modulate the TME by regulating the polarization of TAMs (77) (Figure 3B). The authors found that miR-145 secreted by colorectal cancer cells via MVs is taken up by TAMs, targets histone deacetylase 11 (HDAC11) and TLR4, enhances the production of IL-10, promotes the polarization of TAMs to M2-like macrophages, and promotes tumor progression. In addition, MV-treated macrophages cause significant enlargement of the tumor (77).
The crosstalk of specific miRNAs between different immune and tumor cells has been widely demonstrated in the TME (all the representative miRNAs mentioned in the text related to the immune response in the TME are summarized in Tables 3, 4). Thus, miRNAs are potential targets in immunotherapy, and modulating specific miRNA expression in immune or tumor cells or both may have a significant effect on tumor defense.
Table 3.
Representative microRNAs (miRNAs) expressed in immune cells related to immune response in tumor microenvironment (TME).
| Tumor-derived/tumor-derived factors induced miRNAs | Host cells | Immune-related roles in the TME | References |
|---|---|---|---|
| miR-17 | Th1 cells | Inhibits the differentiation of iTregs, enhances Th1 response to tumor cells | (20) |
| miR-19b, 24, 181 | Th1 cells | Promote the proliferation of Th1 cells and production of IFN-γ, inhibit the differentiation of iTregs, inhibit tumor progression | (20) |
| miR-21 | Tregs | Reduces the proliferation of Tregs, abrogates immunosuppressive capacity | (26) |
| miR-31, 210, 17-92 cluster | Tregs | Promote the expression of Foxp3 and IL-10, enhance immunosuppressive function | (27) |
| (28) | |||
| (29) | |||
| miR-29, 146a, 155 | CD8+T cells | Promote/inhibit the production of IFN-γ, affect tumor growth | (31) |
| (32) | |||
| miR-17-92 cluster, 23a | CD8+T cells | Promote/inhibit the production of perforin/granzyme B, regulate tumor progression | (34) |
| (35) | |||
| miR-155 et al | NKs | Promote/inhibit the production of IFN-γ, affect tumor growth | (13) |
| miR-150 et al | NKs | Inhibit the production of perforin/granzyme B, promote tumor progression | (56) |
| miR-182 | NKs | Promotes the expression of activating receptors NKG2D, enhances cytotoxicity of NKs | (38) |
| miR-22 | DCs | Inhibits polarization of Th17, downregulates CD40, CD80, CD86, decreases the antitumor activity of DCs | (59) |
| miR-155, 301 | DCs | Promote DCs maturation, enhance the capacity of T cell activation, abrogate tumor progression | (60) (62) (61) |
| miR-494, 21, 155 | MDSCs | Upregulate ARG1 and iNOS, enhance immunosuppression | (67) (68) |
| miR-17-5p, 20a | MDSCs | Reduce the production of ROS, inhibit the immunosuppressive function of MDSCs | (69) |
| miR-146a, 155 et al | TAMs | Regulate the polarization of TAMs to M1/M2 phenotype, change the fate of tumor cells | (71) (73) |
Table 4.
Representative tumor-derived/tumor-derived factors induced microRNAs (miRNAs) related to immune response in tumor microenvironment (TME).
| Tumor-derived/tumor -derived factors induced miRNAs | Host cells | Immune-related roles in the TME | References |
|---|---|---|---|
| miR-24-3p | Th1 cells | Affects the secretion of cytokines such as IFN-γ, reduces the antitumor effect | (22) |
| miR-214 | Tregs | Increases the secretion of IL-10 by Tregs, promotes tumor growth | (30) |
| miR-491 | CD8+ T cells | Decreases proliferation, IFN-γ production, increases apoptosis of CD8+ T cells, enhances tumor immune escape | (36) |
| miR-23a | NKs | Reduces IFN-γ production and CD107a expression of NKs, enhances immunosuppressive effect | (58) |
| miR-212-3p, 203 | DCs | Decrease expression of MHC-II, inhibit maturation and differentiation, induce immune tolerance of DCs | (63) (64) |
| miR-200c, 494 | MDSCs | Promote MDSCs expansion and accumulation of MDSCs in tumor tissues, facilitate tumor invasion and metastasis | (67) (70) |
| miR-145 | TAMs | Enhances the production of IL-10 and the polarization of TAMs to M2-like macrophages, promotes tumor progression | (77) |
miRNAs as modulators between CAEs and tumor cells
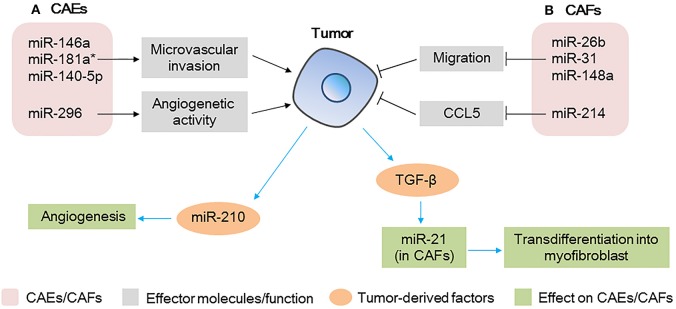
Endothelial cells are important components of tumor stroma. They line the interior surface of blood vessels and lymphatic vessels and in TME, the unregulated growth and migration of CAEs contribute considerably to angiogenesis and regulation of tumor metastasis (78). Altered expression of miRNAs in CAEs also affect tumor progression as shown in Figure 4A. In a HCC model, when endothelial cells were co-cultured with HCC cells, miR-146a, miR-181a*, and miR-140-5p were significantly upregulated and miR-302c was downregulated. Among these miRNAs, the upregulation of miR-146a was found to promote CAEs proliferation, vascularization, and tumor growth (79). Mechanism proof studies revealed that miR-146a promoted the expression of platelet-derived growth factor receptor α (PDGFRA) in CAEs, which was mediated by BRCA1 (79). Moreover, overexpression of PDGFRA in the CAEs of HCC tissues was found to be associated with microvascular invasion and predicted a poorer prognosis, indicating that miR-146a plays a key role in regulating the angiogenetic activity of CAEs in HCC (79). Other miRNAs such as miR-296 were also found to be upregulated in CAEs in a glioma model, which was found to promote angiogenesis by increasing endothelial cells and finally promote tumor growth (80).
Figure 4.
microRNAs (miRNAs) act as modulators between cancer-associated endothelial cells (CAEs)/cancer-associated fibroblasts (CAFs) and tumor cells (A) miRNAs expressed in CAEs modulate tumor progression by regulating the microvascular invasion and angiogenesis activity of CAEs; tumor-derived miRNAs affect the angiogenesis capacity of CAEs. (B) miRNAs expressed in CAFs modulate tumor progression by regulating the migration and chemokine production; tumor-derived factors affect miRNAs expression in CAFs, further affect the transdifferentiation of myofibroblasts.
miRNAs derived from tumor cells could also affect angiogenesis of CAEs as shown in Figure 4A. In a study of patients with HCC, a high level of miR-210-3p (miR-210) was detected in the exosomes isolated from the sera of the patients. Further studies demonstrated that HCC cell-derived exosomal miR-210 could be delivered to CAEs, enhancing angiogenesis (81). Subsequent in vivo studies revealed that subcutaneous tumor xenografts treated with HCC cells-derived exosomal miR-210 showed much more vessels and a larger tumor volume than control group without exosomal miRNA (81). These findings revealed that miR-210 may be used as a potential therapeutic target in anti-HCC therapy.
Endothelial cells are critical for angiogenesis and regulating tumor metastasis, deregulated miRNAs expression in CAEs do affect the tumor progression, this reminds us that targeting miRNAs in CAEs could be a new approach for cancer therapy.
miRNAs as modulators between CAFs and tumor cells
In the TME, fibroblasts regulate angiogenesis and metastasis of tumor cells, and miRNAs are notable key regulators of the tumor promoting function of CAFs as shown in Figure 4B. A miRNA array analysis of CAFs in breast cancer showed that six miRNAs were significantly downregulated, consisting of miR-7f, miR-7g, miR-15b, miR-26b, miR-30b, and miR-107. Furthermore, among these miRNAs, miR-26b was the most highly deregulated, and its downregulation in CAFs promoted the migration of fibroblasts and further promoted the invasion of breast cancer cells (82). Similarly, miR-31 and miR148a were also found to be downregulated in CAFs in endometrial cancer, which finally promoted tumor invasiveness and growth (83, 84). Moreover, miR-214 in CAFs was reported to be downregulated in patients with ovarian cancer and in addition, the expression of the pro-tumorgenetic chemokine CCL5 was enhanced, finally promoting the invasion and growth of ovarian cancer cells (85).
Tumor-derived factors such as TGF-β could also regulate the expression of miRNAs in CAFs, further affecting tumor progression as shown in Figure 4B. For example, miR-21 was reported to be upregulated in CAFs by colorectal tumor-derived TGF-β, increased expression of miR-21 was shown to upregulate α-SMA, which promotes CAFs transdifferentiation into myofibroblasts, resulting in increased proliferative capacity of colorectal cancer (86). TGF-β was also shown to upregulate CAFs miR-143 in gastric cancer, which finally promoted tumorigenesis by enhancing the expression of collagen type III (87).
Taken together, these data highlight the importance of miRNAs deregulation in CAEs and CAFs, and identify a potential application for stromal miRNAs as biomarkers in cancer.
A perspective on miRNAs in cancer therapy
Recent and ongoing investigations have enhanced our knowledge of the function of miRNAs in tumor biology and immune cell development. miRNAs can affect tumor progression by regulating gene expression in tumor cells or modulating the function of immune cells in the TME. Because of their multiple functions in physiological processes, miRNAs are considered as potential biomarkers in diagnostics and prognosis of various cancers (7). In a study of 391 patients with non-small cell lung cancer (NSCLC), serum miRNAs were shown to serve as predictors and biomarkers of survival for patients with advanced NSCLC (88).
miRNAs are also proposed to be therapeutic targets. miRNAs that act as suppressors of tumorigenesis need to be overexpressed, while those inducing tumorigenesis need to be silenced. The current strategy to overexpress or inhibit miRNAs uses the delivery of synthetic oligoribonucleotides, which mimic the native miRNA duplex or are antisense RNA. Different types of vehicles have been synthesized as carriers of miRNA mimics and antagonists, including liposomes, polymers, nanoparticles, and viral agents (89); they have been found to improve the efficiency and specificity of the systemic delivery of miRNA (90–92). We have indicated that several examples showing tumor-derived MVs could modulate the immune response. Thus, cell-derived exosomes containing immune-related miRNAs might be used as therapeutic agents to enhance antitumor immunity.
While many studies indicate the possible clinical application of miRNAs (93), clinical trials have tested the efficacy of miRNA-based cancer therapeutics. MRX34, a synthetic miR-34a, was used in a phase I clinical trial in patients with primary liver cancer and liver metastasis in 2013. In the study, miR-34a, a tumor suppressor downstream of p53, was overexpressed by loading it into liposomal nanoparticles (94). Although it originally seemed promising, the study was halted in 2016 because of multiple adverse immune-related events. In addition, many ASOs have also been tested in clinical trials. These therapies are RNA-based (not miRNA-based); they do not knockdown or overexpress miRNAs but target oncogenes such as Bcl-2 (NCT00285103) in CLL, STAT3 (NCT01563302) in DLBCL and lymphoma, and MYC (NCT02314052) in HCC. These therapeutics have already reached phase I/II (https://clinicaltrials.gov/).
Although miRNA-based therapies provide hope for their clinical application, some issues need to be considered: (1) miRNAs target molecules in both normal and cancer cells, and their efficacy and safety need to be tested; (2) miRNA-based agents are easily degraded and, therefore, new approaches are necessary to enhance their stability and prevent degradation by nucleases; (3) because the therapeutics need to reach target tissues, their half-life has to be long enough; and (4) to avoid miRNA-based therapeutics being taken up by normal cells, proper delivery systems need to be developed.
Conclusion
miRNAs are multifunctional molecules that play essential roles in physiological processes, and the deregulation of miRNAs is often associated with human diseases. In this review, we have discussed the crosstalk of miRNAs between immune cells, CAEs, CAFs, and tumor cells in the TME and have summarized the potential clinical application of miRNA-based therapeutics. While most of the investigations focus on the deregulation of miRNAs in cancer cells, the altered expression of miRNAs in immune cells, CAEs and CAFs in the TME can also affect tumor progression. Thus, the identification of these altered miRNAs may lead to the discovery of novel biomarkers for immunotherapy. Overall, in consideration of the complex functions of miRNAs in different cell types, we believe that in the future, miRNA-based therapeutics will be used to treat a broad range of human diseases.
Author contributions
NY carried out the primary literature search, wrote and revised the manuscript, and created the illustrations. SZ, XPL, YQ, and Y-JL were involved in the preparation and revision of the manuscript. JTC initiated the concept and supervised the writing and revision of the manuscript. All authors read and approved the final manuscript.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Funding. This article was supported by the National Natural Science Foundation of China (Grant Nos. 81571534, 81701563, 81870152, and 81402436), the Science and Technology Department of Jilin province (No. 20180101097JC), the Youth Development Fund of First Hospital of Jilin University (No. JDYY82017024), the Fundamental Research Funds for the Central Universities (No. 2017TD-08), the Program for JLU Science and Technology Innovative Research Team, and Norman Bethune Program of Jilin University (No. 2015413).
References
- 1.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell (2004) 116:281–97. 10.1016/S0092-8674(04)00045-5 [DOI] [PubMed] [Google Scholar]
- 2.Lee RC, Feinbaum RL, Ambros V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell (1993). 75:843–54. [DOI] [PubMed] [Google Scholar]
- 3.Hammond SM. An overview of microRNAs. Adv Drug Deliv Rev. (2015) 87:3–14. 10.1016/j.addr.2015.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lundstrom K. Micro-RNA in disease and gene therapy. Curr Drug Discov Technol. (2011) 82:76–86. 10.2174/157016311795563857 [DOI] [PubMed] [Google Scholar]
- 5.Friedman RC, Farh KKH, Burge CB, Bartel DP. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res. (2008) 19:92–105. 10.1101/gr.082701.108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tüfekci KU, Öner MG, Meuwissen RLJ, Genç S. The role of MicroRNAs in human Diseases. Methods Mol Biol. (2014) 1107:33–50. 10.1007/978-1-62703-748-83 [DOI] [PubMed] [Google Scholar]
- 7.Di Leva G, Garofalo M, Croce CM. MicroRNAs in cancer. Annu Rev Pathol. (2014) 9:287–314. 10.1146/annurev-pathol-012513-104715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Calin GA, Dumitru CD, Shimizu M, Bichi R, Zupo S, Noch E, et al. Frequent deletions and down-regulation of micro- RNA genes miR15 and miR16 at 13q14 in chronic lymphocytic leukemia. Proc Natl Acad Sci USA (2002) 99:15524–9. 10.1073/pnas.242606799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lawrie CH, Gal S, Dunlop HM, Pushkaran B, Liggins AP, Pulford K, et al. Detection of elevated levels of tumour-associated microRNAs in serum of patients with diffuse large B-cell lymphoma. Br J Haematol. (2008) 141:672–5. 10.1111/j.1365-2141.2008.07077.x [DOI] [PubMed] [Google Scholar]
- 10.Adams BD, Parsons C, Walker L, Zhang WC, Slack FJ. Targeting noncoding RNAs in disease. J Clin Invest. (2017) 127:761–71. 10.1172/JCI84424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rodriguez A, Vigorito E, Clare S, Warren MV, Couttet P, Soond DR, et al. Requirement of bic/microRNA-155 for normal immune function. Science (2007) 316:608–11. 10.1126/science.1139253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lu LF, Thai TH, Calado DP, Chaudhry A, Kubo M, Tanaka K, et al. Foxp3-dependent microRNA155 confers competitive fitness to regulatory T cells by targeting SOCS1 protein. Immunity (2009) 30:80–91. 10.1016/j.immuni.2008.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Trotta R, Chen L, Ciarlariello D, Josyula S, Mao C, Costinean S, et al. miR-155 regulates IFN-gamma production in natural killer cells. Blood (2012) 119:3478–85. 10.1182/blood-2011-12-398099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Trotta R, Chen L, Costinean S, Josyula S, Mundy-Bosse BL, Ciarlariello D, et al. Overexpression of miR-155 causes expansion, arrest in terminal differentiation and functional activation of mouse natural killer cells. Blood (2013) 121:3126–34. 10.1182/blood-2012-12-467597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang J, Iwanowycz S, Yu F, Jia X, Leng S, Wang Y, et al. microRNA-155 deficiency impairs dendritic cell function in breast cancer. Oncoimmunology (2016) 5:e1232223. 10.1080/2162402X.2016.1232223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen S, Zhang Y, Kuzel TM, Zhang B. Regulating tumor myeloid-derived suppressor cells by microRNAs. Cancer Cell Microenviron. (2015) 2:e637. 10.14800/ccm.637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.He M, Xu Z, Ding T, Kuang DM, Zheng L. MicroRNA-155 regulates inflammatory cytokine production in tumor-associated macrophages via targeting C/EBPbeta. Cell Mol Immunol. (2009) 6:343–35. 10.1038/cmi.2009.45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baltimore D, Boldin MP, O'Connell RM, Rao DS, Taganov KD. MicroRNAs: new regulators of immune cell development and function. Nat Immunol. (2008) 9:839–45. 10.1038/ni.f.209 [DOI] [PubMed] [Google Scholar]
- 19.Baumjohann D, Ansel KM. MicroRNA-mediated regulation of T helper cell differentiation and plasticity. Nat Rev Immunol. (2013) 139:666–78. 10.1038/nri3494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jiang S, Li C, Olive V, Lykken E, Feng F, Sevilla J, et al. Molecular dissection of the miR-17–92 cluster's critical dual roles in promoting Th1 responses and preventing inducible Treg differentiation. Blood (2011) 118:5487–97. 10.1182/blood-2011-05-355644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fayyad-Kazan H, Hamade E, Rouas R, Najar M, Fayyad-Kazan M, El Zein N, et al. Downregulation of microRNA-24 and−181 parallels the upregulation of IFN-gamma secreted by activated human CD4 lymphocytes. Hum Immunol. (2014) 75:677–85. 10.1016/j.humimm.2014.01.007 [DOI] [PubMed] [Google Scholar]
- 22.Ye SB, Li ZL, Luo DH, Huang BJ, Chen YS, Zhang XS, et al. Tumor-derived exosomes promote tumor progression and T-cell dysfunction through the regulation of enriched exosomal microRNAs in human nasopharyngeal carcinoma. Oncotarget (2014) 5:5439–52. 10.18632/oncotarget.2118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang B, Zhao J, Lei Z, Shen S, Li D, Shen GX, et al. miR-142–3p restricts cAMP production in CD4+CD25- T cells and CD4+CD25+ TREG cells by targeting AC9 mRNA. EMBO Rep. (2009) 10:180–5. 10.1038/embor.2008.224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lu LF, Boldin MP, Chaudhry A, Lin LL, Taganov KD, Hanada T, et al. Function of miR-146a in controlling treg cell-mediated regulation of Th1 responses. Cell (2010) 142:914–29. 10.1016/j.cell.2010.08.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Qin A, Wen Z, Zhou Y, Li Y, Li Y, Luo J, et al. MicroRNA-126 regulates the induction and function of CD4(+) Foxp3(+) regulatory T cells through PI3K/AKT pathway. J Cell Mol Med. (2013) 17:252–64. 10.1111/jcmm.12003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hu Y, Wang C, Li Y, Zhao J, Chen C, Zhou Y, et al. MiR-21 controls in situ expansion of CCR6(+) regulatory T cells through PTEN/AKT pathway in breast cancer. Immunol Cell Biol. (2015) 93:753–64. 10.1038/icb.2015.37 [DOI] [PubMed] [Google Scholar]
- 27.Rouas R, Fayyad-Kazan H, El Zein N, Lewalle P, Rothe F, Simion A, et al. Human natural Treg microRNA signature: role of microRNA-31 and microRNA-21 in FOXP3 expression. Eur J Immunol. (2009) 396:1608–18. 10.1002/eji.200838509 [DOI] [PubMed] [Google Scholar]
- 28.Zhao M, Wang LT, Liang GP, Zhang P, Deng XJ, Tang Q, et al. Up-regulation of microRNA-210 induces immune dysfunction via targeting FOXP3 in CD4(+) T cells of psoriasis vulgaris. Clin Immunol. (2014) 150:22–30. 10.1016/j.clim.2013.10.009 [DOI] [PubMed] [Google Scholar]
- 29.de Kouchkovsky D, Esensten JH, Rosenthal WL, Morar MM, Bluestone JA, Jeker LT. microRNA-17–92 regulates IL-10 production by regulatory T cells and control of experimental autoimmune encephalomyelitis. J Immunol. (2013) 191:1594–605. 10.4049/jimmunol.1203567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yin Y, Cai X, Chen X, Liang H, Zhang Y, Li J, et al. Tumor-secreted miR-214 induces regulatory T cells: a major link between immune evasion and tumor growth. Cell Res. (2014) 24:1164–80. 10.1038/cr.2014.121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ma F, Xu S, Liu X, Zhang Q, Xu X, Liu M, et al. The microRNA miR-29 controls innate and adaptive immune responses to intracellular bacterial infection by targeting interferon-gamma. Nat Immunol. (2011) 12:861–9. 10.1038/ni.2073 [DOI] [PubMed] [Google Scholar]
- 32.Huffaker TB, Hu R, Runtsch MC, Bake E, Chen X, Zhao J, et al. Epistasis between microRNAs 155 and 146a during T cell-mediated antitumor immunity. Cell Rep. (2012) 2:1697–709. 10.1016/j.celrep.2012.10.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dudda JC, Salaun B, Ji Y, Palmer DC, Monnot GC, Merck E, et al. MicroRNA-155 is required for effector CD8+ T cell responses to virus infection and cancer. Immunity (2013) 38:742–53. 10.1016/j.immuni.2012.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Khan AA, Penny LA, Yuzefpolskiy Y, Sarkar S, Kalia V. MicroRNA-17~92 regulates effector and memory CD8 T-cell fates by modulating proliferation in response to infections. Blood (2013) 121:4473–83. 10.1182/blood-2012-06-435412 [DOI] [PubMed] [Google Scholar]
- 35.Lin R, Chen L, Chen G, Hu C, Jiang S, Sevilla J, et al. Targeting miR-23a in CD8+ cytotoxic T lymphocytes prevents tumor-dependent immunosuppression. J Clin Invest. (2014) 124:5352–67. 10.1172/JCI76561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yu T, Zuo QF, Gong L, Wang LN, Zou QM, Xiao B. MicroRNA-491 regulates the proliferation and apoptosis of CD8(+) T cells. Sci Rep. (2016) 6:30923. 10.1038/srep30923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Espinoza JL, Takami A, Yoshioka K, Nakata K, Sato T, Kasahara Y, et al. Human microRNA-1245 down-regulates the NKG2D receptor in natural killer cells and impairs NKG2D-mediated functions. Haematologica (2012) 97:1295–303. 10.3324/haematol.2011.058529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Abdelrahman MM, Fawzy IO, Bassiouni AA, Gomaa AI, Esmat G, Waked I, et al. Enhancing NK cell cytotoxicity by miR-182 in hepatocellular carcinoma. Hum Immunol. (2016) 77:667–73. 10.1016/j.humimm.2016.04.020 [DOI] [PubMed] [Google Scholar]
- 39.Li Q, Johnston N, Zheng X, Wang H, Zhang X, Gao D, et al. miR-28 modulates exhaustive differentiation of T cells through silencing programmed cell death-1 and regulating cytokine secretion. Oncotarget (2016) 7:53735–50. 10.18632/oncotarget.10731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ma Y, Gong J, Liu Y, Guo W, Jin B, Wang X, et al. MicroRNA-30c promotes natural killer cell cytotoxicity via up-regulating the expression level of NKG2D. Life Sci. (2016) 151:174–81. 10.1016/j.lfs.2016.03.012 [DOI] [PubMed] [Google Scholar]
- 41.Wei J, Nduom EK, Kong LY, Hashimoto Y, Xu S, Gabrusiewicz K, et al. MiR-138 exerts anti-glioma efficacy by targeting immune checkpoints. Neuro Oncol. (2016) 18:639–48. 10.1093/neuonc/nov292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang J, Liu R, Deng Y, Qian J, Lu Z, Wang Y, et al. MiR-15a/16 deficiency enhances anti-tumor immunity of glioma-infiltrating CD8+ T cells through targeting mTOR. Int J Cancer (2017) 141:2082–92. 10.1002/ijc.30912 [DOI] [PubMed] [Google Scholar]
- 43.Chen L, Gibbons DL, Goswami S, Cortez MA, Ahn Y-H, Byers LA, et al. Metastasis is regulated via microRNA-200/ZEB1 axis control of tumour cell PD-L1 expression and intratumoral immunosuppression. Nat Commun. (2014) 5:5241. 10.1038/ncomms6241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhu J, Chen L, Zou L, Yang P, Wu R, Mao Y, et al. MiR-20b,−21, and−130b inhibit PTEN expression resulting in B7-H1 over-expression in advanced colorectal cancer. Hum Immunol. (2014) 75:348–53. 10.1016/j.humimm.2014.01.006 [DOI] [PubMed] [Google Scholar]
- 45.Pandya D, Mariani M, He S, Andreoli M, Spennato M, Dowell-Martino C, et al. Epstein-barr virus microRNA expression increases aggressiveness of solid malignancies. PLoS ONE (2015) 10:e0136058. 10.1371/journal.pone.0136058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang X, Li J, Dong K, Lin F, Long M, Ouyang Y, et al. Tumor suppressor miR-34a targets PD-L1 and functions as a potential immunotherapeutic target in acute myeloid leukemia. Cell Signal (2015) 27:443–52. 10.1016/j.cellsig.2014.12.003 [DOI] [PubMed] [Google Scholar]
- 47.Xu S, Tao Z, Hai B, Liang H, Shi Y, Wang T, et al. miR-424(322) reverses chemoresistance via T-cell immune response activation by blocking the PD-L1 immune checkpoint. Nat Commun. (2016) 7:11406. 10.1038/ncomms11406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jia L, Xi Q, Wang H, Zhang Z, Liu H, Cheng Y, et al. miR-142–5p regulates tumor cell PD-L1 expression and enhances anti-tumor immunity. Biochem Biophys Res Commun. (2017) 488:425–31. 10.1016/j.bbrc.2017.05.074 [DOI] [PubMed] [Google Scholar]
- 49.Smyth MJ, Hayakawa Y, Takeda K, Yagita H. New aspects of natural-killer-cell surveillance and therapy of cancer. Nat Rev Cancer (2002) 2:850–61. 10.1038/nrc928 [DOI] [PubMed] [Google Scholar]
- 50.Ni F, Guo C, Sun R, Fu B, Yang Y, Wu L, et al. MicroRNA transcriptomes of distinct human NK cell populations identify miR-362–5p as an essential regulator of NK cell function. Sci Rep. (2015) 5:9993. 10.1038/srep09993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cichocki F, Felices M, McCullar V, Presnell SR, Al-Attar A, Lutz CT, et al. Cutting edge: microRNA-181 promotes human NK cell development by regulating Notch signaling. J Immunol. (2011) 187:6171–5. 10.4049/jimmunol.1100835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sullivan RP, Leong JW, Schneider SE, Ireland AR, Berrien-Elliott MM, Singh A, et al. MicroRNA-15/16 antagonizes Myb to control NK cell maturation. J Immunol. (2015) 195:2806–17. 10.4049/jimmunol.1500949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fehniger TA, Wylie T, Germino E, Leong JW, Magrini VJ, Koul S, et al. Next-generation sequencing identifies the natural killer cell microRNA transcriptome. Genome Res. (2010) 20:1590–604. 10.1101/gr.107995.110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kim TD, Lee SU, Yun S, Sun HN, Lee SH, Kim JW, et al. Human microRNA-27a* targets Prf1 and GzmB expression to regulate NK-cell cytotoxicity. Blood (2011) 118:5476–86. 10.1182/blood-2011-04-347526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang P, Gu Y, Zhang Q, Han Y, Hou J, Lin L, et al. Identification of resting and type I IFN-activated human NK cell miRNomes reveals microRNA-378 and microRNA-30e as negative regulators of NK cell cytotoxicity. J Immunol. (2012) 189:211–21. 10.4049/jimmunol.1200609 [DOI] [PubMed] [Google Scholar]
- 56.Kim N, Kim M, Yun S, Doh J, Greenberg PD, Kim TD, et al. MicroRNA-150 regulates the cytotoxicity of natural killers by targeting perforin-1. J Allergy Clin Immunol. (2014) 134:195–203. 10.1016/j.jaci.2014.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Espinoza JL, Nguyen VH, Ichimura H, Pham TT, Nguyen CH, Pham TV, et al. A functional polymorphism in the NKG2D gene modulates NK-cell cytotoxicity and is associated with susceptibility to human papilloma virus-related cancers. Sci Rep. (2016) 6:39231. 10.1038/srep39231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Berchem G, Noman MZ, Bosseler M, Paggetti J, Baconnais S, Le Cam E, et al. Hypoxic tumor-derived microvesicles negatively regulate NK cell function by a mechanism involving TGF-beta and miR23a transfer. Oncoimmunology (2016) 5:e1062968. 10.1080/2162402X.2015.1062968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liang X, Liu Y, Mei S, Zhang M, Xin J, Zhang Y, et al. MicroRNA-22 impairs anti-tumor ability of dendritic cells by targeting p38. PLoS ONE (2015) 10:e0121510. 10.1371/journal.pone.0121510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dunand-Sauthier I, Santiago-Raber ML, Capponi L, Vejnar CE, Schaad O, Irla M, et al. Silencing of c-Fos expression by microRNA-155 is critical for dendritic cell maturation and function. Blood (2011) 117:4490–500. 10.1182/blood-2010-09-308064 [DOI] [PubMed] [Google Scholar]
- 61.Pyfferoen L, Mestdagh P, Vergote K, De Cabooter N, Vandesompele J, Lambrecht BN, et al. Lung tumours reprogram pulmonary dendritic cell immunogenicity at the microRNA level. Int J Cancer (2014) 135:2868–77. 10.1002/ijc.28945 [DOI] [PubMed] [Google Scholar]
- 62.Cubillos-Ruiz JR, Baird JR, Tesone AJ, Rutkowski MR, Scarlett UK, Camposeco-Jacobs AL, et al. Reprogramming tumor-associated dendritic cells in vivo using miRNA mimetics triggers protective immunity against ovarian cancer. Cancer Res. (2012) 72:1683–93. 10.1158/0008-5472.CAN-11-3160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ding G, Zhou L, Qian Y, Fu M, Chen J, Chen J, et al. Pancreatic cancer-derived exosomes transfer miRNAs to dendritic cells and inhibit RFXAP expression via miR-212–3p. Oncotarget (2015) 6:29877–88. 10.18632/oncotarget.4924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhou M, Chen J, Zhou L, Chen W, Ding G, Cao L. Pancreatic cancer derived exosomes regulate the expression of TLR4 in dendritic cells via miR-203. Cell Immunol. (2014) 292:65–9. 10.1016/j.cellimm.2014.09.004 [DOI] [PubMed] [Google Scholar]
- 65.Bronte V, Zanovello P. Regulation of immune responses by L-arginine metabolism. Nat Rev Immunol. (2005) 5:641–54. 10.1038/nri1668 [DOI] [PubMed] [Google Scholar]
- 66.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. (2009) 9:162–74. 10.1038/nri2506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liu Y, Lai L, Chen Q, Song Y, Xu S, Ma F, et al. MicroRNA-494 is required for the accumulation and functions of tumor-expanded myeloid-derived suppressor cells via targeting of PTEN. J Immunol. (2012) 188:5500–10. 10.4049/jimmunol.1103505 [DOI] [PubMed] [Google Scholar]
- 68.Li L, Zhang J, Diao W, Wang D, Wei Y, Zhang CY, et al. MicroRNA-155 and MicroRNA-21 promote the expansion of functional myeloid-derived suppressor cells. J Immunol. (2014) 192:1034–43. 10.4049/jimmunol.1301309 [DOI] [PubMed] [Google Scholar]
- 69.Zhang M, Liu Q, Mi S, Liang X, Zhang Z, Su X, et al. Both miR-17–5p and miR-20a alleviate suppressive potential of myeloid-derived suppressor cells by modulating STAT3 expression. J Immunol. (2011) 186:4716–24. 10.4049/jimmunol.1002989 [DOI] [PubMed] [Google Scholar]
- 70.Mei S, Xin J, Liu Y, Zhang Y, Liang X, Su X, et al. MicroRNA-200c promotes suppressive potential of myeloid-derived suppressor cells by modulating PTEN and FOG2 expression. PLoS ONE (2015) 10:e0135867. 10.1371/journal.pone.0135867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cai X, Yin Y, Li N, Zhu D, Zhang J, Zhang CY, et al. Re-polarization of tumor-associated macrophages to pro-inflammatory M1 macrophages by microRNA-155. J Mol Cell Biol. (2012) 4:341–3. 10.1093/jmcb/mjs044 [DOI] [PubMed] [Google Scholar]
- 72.Yang J, Zhang Z, Chen C, Liu Y, Si Q, Chuang TH, et al. MicroRNA-19a-3p inhibits breast cancer progression and metastasis by inducing macrophage polarization through downregulated expression of Fra-1 proto-oncogene. Oncogene (2014) 33:3014–23. 10.1038/onc.2013.258 [DOI] [PubMed] [Google Scholar]
- 73.Li Y, Zhao L, Shi B, Ma S, Xu Z, Ge Y, et al. Functions of miR-146a and miR-222 in tumor-associated macrophages in breast cancer. Sci Rep. (2015) 5:18648. 10.1038/srep18648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sonda N, Simonato F, Peranzoni E, Cali B, Bortoluzzi S, Bisognin A, et al. miR-142–3p prevents macrophage differentiation during cancer-induced myelopoiesis. Immunity (2013) 386:1236–49. 10.1016/j.immuni.2013.06.004 [DOI] [PubMed] [Google Scholar]
- 75.Chaudhuri AA, So AY-L, Sinha N, Gibson WSJ, Taganov KD, O'Connell RM, et al. MicroRNA-125b potentiates macrophage activation. J Immunol. (2011) 187:5062–8. 10.4049/jimmunol.1102001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Squadrito ML, Pucci F, Magri L, Moi D, Gilfillan GD, Ranghetti A, et al. miR-511–3p modulates genetic programs of tumor-associated macrophages. Cell Rep. (2012) 1:141–54. 10.1016/j.celrep.2011.12.005 [DOI] [PubMed] [Google Scholar]
- 77.Shinohara H, Kuranaga Y, Kumazaki M, Sugito N, Yoshikawa Y, Takai T, et al. Regulated Polarization of tumor-associated macrophages by miR-145 via colorectal cancer-derived extracellular vesicles. J Immunol. (2017) 199:1505–15. 10.4049/jimmunol.1700167 [DOI] [PubMed] [Google Scholar]
- 78.Junttila MR, de Sauvage FJ. Influence of tumour micro-environment heterogeneity on therapeutic response. Nature (2013) 501:346–54. 10.1038/nature12626 [DOI] [PubMed] [Google Scholar]
- 79.Zhu K, Pan Q, Zhang X, Kong LQ, Fan J, Dai Z, et al. MiR-146a enhances angiogenic activity of endothelial cells in hepatocellular carcinoma by promoting PDGFRA expression. Carcinogenesis (2013) 34:2071–9. 10.1093/carcin/bgt160 [DOI] [PubMed] [Google Scholar]
- 80.Plummer PN, Freeman R, Taft RJ, Vider J, Sax M, Umer BA, et al. MicroRNAs regulate tumor angiogenesis modulated by endothelial progenitor cells. Cancer Res. (2013) 73:341–52. 10.1158/0008-5472.CAN-12-0271 [DOI] [PubMed] [Google Scholar]
- 81.Lin XJ, Fang JH, Yang XJ, Zhang C, Yuan Y, Zheng L, et al. Hepatocellular carcinoma cell-secreted exosomal MicroRNA-210 promotes angiogenesis in vitro and in vivo. Mol Ther Nucleic Acids (2018) 11:243–52. 10.1016/j.omtn.2018.02.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Verghese ET, Drury R, Green CA, Holliday DL, Lu X, Nash C, et al. MiR-26b is down-regulated in carcinoma-associated fibroblasts from ER-positive breast cancers leading to enhanced cell migration and invasion. J Pathol. (2013) 231:388–99. 10.1002/path.4248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Aprelikova O, Yu X, Palla J, Wei BR, John S, Yi M, et al. The role of miR-31 and its target gene SATB2 in cancer-associated fibroblasts. Cell Cycle (2010) 9:4387–98. 10.4161/cc.9.21.13674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Aprelikova O, Palla J, Hibler B, Yu X, Greer YE, Yi M, et al. Silencing of miR-148a in cancer-associated fibroblasts results in WNT10B-mediated stimulation of tumor cell motility. Oncogene (2013) 32:3246–53. 10.1038/onc.2012.351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mitra AK, Zillhardt M, Hua Y, Tiwari P, Murmann AE, Peter ME, et al. MicroRNAs reprogram normal fibroblasts into cancer-associated fibroblasts in ovarian cancer. Cancer Discov. (2012) 2:1100–8. 10.1158/2159-8290.CD-12-0206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bullock MD, Pickard KM, Nielsen BS, Sayan AE, Jenei V, Mellone M, et al. Pleiotropic actions of miR-21 highlight the critical role of deregulated stromal microRNAs during colorectal cancer progression. Cell Death Dis. (2013) 4:e684. 10.1038/cddis.2013.213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Naito Y, Sakamoto N, Oue N, Yashiro M, Sentani K, Yanagihara K, et al. MicroRNA-143 regulates collagen type III expression in stromal fibroblasts of scirrhous type gastric cancer. Cancer Sci. (2014) 105:228–35. 10.1111/cas.12329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wang Y, Gu J, Roth JA, Hildebrandt MA, Lippman SM, Ye Y, et al. Pathway-based serum microRNA profiling and survival in patients with advanced stage non-small cell lung cancer. Cancer Res. (2013) 73:4801–9. 10.1158/0008-5472.CAN-12-3273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Dougan M, Dranoff G. Immune therapy for cancer. Annu Rev Immunol. (2009) 27:83–117. 10.1146/annurev.immunol.021908.132544 [DOI] [PubMed] [Google Scholar]
- 90.Verma IM, Weitzman MD. Gene therapy: twenty-first century medicine. Annu Rev Biochem. (2005) 74:711–38. 10.1146/annurev.biochem.74.050304.091637 [DOI] [PubMed] [Google Scholar]
- 91.Bader AG. miR-34 - a microRNA replacement therapy is headed to the clinic. Front Genet. (2012) 3:120. 10.3389/fgene.2012.00120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Qian X, Long L, Shi Z, Liu C, Qiu M, Sheng J, et al. Star-branched amphiphilic PLA-b-PDMAEMA copolymers for co-delivery of miR-21 inhibitor and doxorubicin to treat glioma. Biomaterials (2014) 35:2322–35. 10.1016/j.biomaterials.2013.11.039 [DOI] [PubMed] [Google Scholar]
- 93.Chakraborty C, Sharma AR, Sharma G, Doss CGP, Lee SS. Therapeutic miRNA and siRNA: moving from bench to clinic as next generation medicine. Mol Ther Nucleic Acids (2017) 8:132–43. 10.1016/j.omtn.2017.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bouchie A. First microRNA mimic enters clinic. Nat Biotechnol. (2013) 31:577. 10.1038/nbt0713-577 [DOI] [PubMed] [Google Scholar]