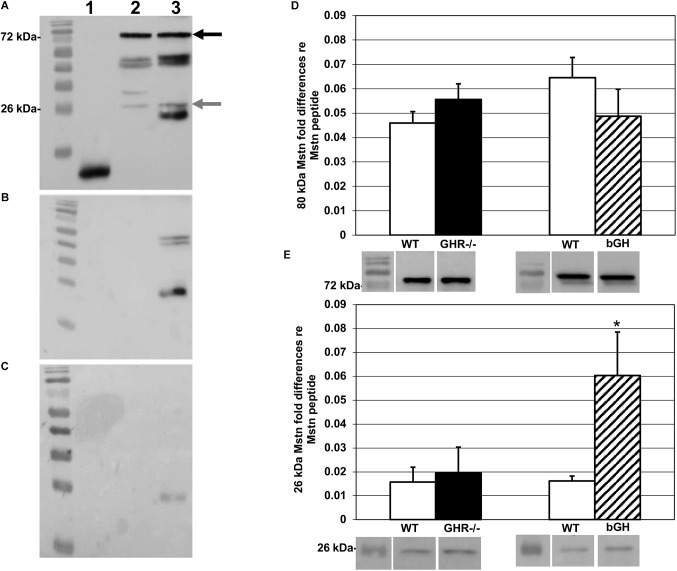
FIGURE 1.
Myostatin protein levels. Western blots probed with the anti-Mstn monoclonal antibody described in the text. In blots A–C, lane 1 contains synthetic Mstn peptide; lane 2, rat triceps surae homogenate; lane 3, mouse triceps surae homogenate. (A) Blot probed with primary and secondary antibody (see text for details). Precursor protein band is indicated by black arrow (80 kDa) and mature protein by gray arrow (26 kDa). (B) Blots probed with secondary antibody alone to test specificity showed non-specific binding in mouse triceps surae homogenate (lane 3). For synthetic Mstn and rat triceps surae (lanes 1 and 2, respectively), we did not find non-specific binding. (C) To reduce non-specific binding, non-conjugated anti-Fab antibody was added to blocking buffer prior to the addition of primary antibody, and this procedure was applied then to all samples. (D) Quantification of levels of precursor Mstn protein, relative to the synthetic Mstn loading control, expressed as fold-change ±SEM. Compared to controls, there was no change in levels of precursor Mstn protein in either group. Representative Western blots are shown under the respective bars. (E) Quantification of levels of mature Mstn protein, relative to the synthetic Mstn loading control, expressed as fold-change ±SEM. There was no change in levels of mature Mstn protein in GHR-/- mice (n = 8) compared to controls. bGH mice (n = 10) expressed significantly more mature Mstn protein compared to controls, ∗p < 0.05. Representative Western blots are shown under the respective bars.