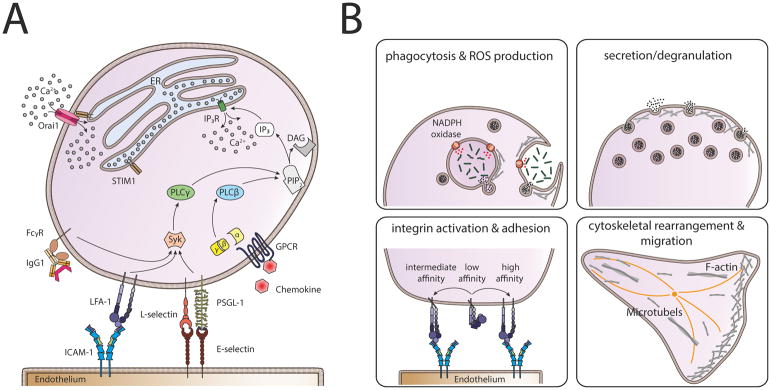
Figure 1. Calcium signaling cascade in neutrophils.
(A) Store operated calcium entry (SOCE) can be initiated either via activation of Fcγ-receptors (FcγRs), β2-integrins, the engagement of P-selectin glycoprotein ligand 1 (PSGL-1) and L-selectin with E-selectin expressed on inflamed endothelium, or via G-protein coupled receptors (GPCRs). Activation of GPCRs leads to dissociation of the G-protein subunits α and βγ and subsequent activation of phospholipase (PLC) β. FcγRs, β2-integrins and PSGL-1/L-selectin signaling in turn, activate PLCγ involving spleen tyrosine kinase (Syk). Both, activated PLCβ and PLCγ convert phosphatidylinositol 4,5 bisphosphate (PIP2) into diacylglycerol (DAG) and inositol-1,4,5 triphosphate (IP3). IP3 binds to and opens the IP3 receptor (IP3R) in the membrane of the endoplasmic reticulum (ER) which results in Ca2+ flux out of the ER into the cytoplasm via IP3R. Upon store depletion, the Ca2+ sensor stromal interaction molecule 1 (STIM1) translocates to PM-ER-junctions and activates Orai1, the predominant Ca2+ release activated Ca2+ (CRAC) channel in neutrophils. This allows the entry of extracellular Ca2+ into the neutrophil, exerting a variety of functions, including (B) phagocytosis, ROS production, secretion and degranulation of vesicles, activation of β2-integrins and cytoskeletal rearrangement leading to polarization and migration.