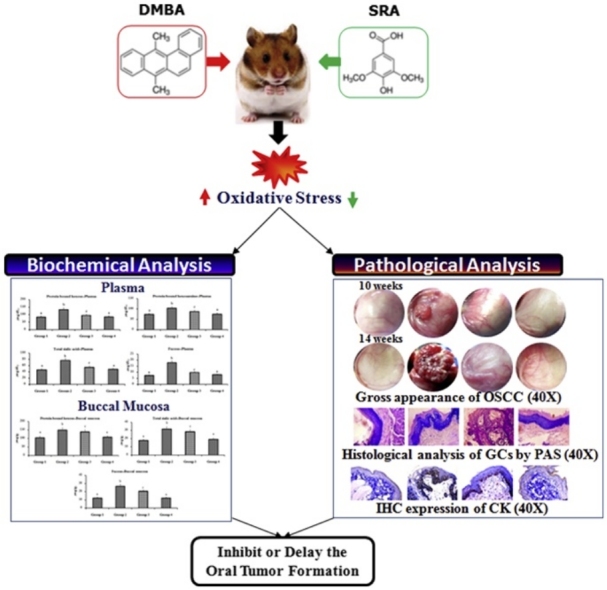
Graphical abstract
Keywords: Syringic acid; Glycoconjugates; 7,12-dimethylbenz(a)anthracene; Oral cancer; Cytokeratin
Highlights
-
•
DMBA induced hamster is a standard model to test oral cancer.
-
•
SRA mediate modulation of GPs expression.
-
•
SRA indicates membrane stabilizing effect during neoplastic alteration.
-
•
SRA inhibits normal GPs separation and regulates glycosyl transferase activity.
Abstract
Syringic acid (SRA) is an excellent anti-oxidant and anti-cancer property in various in vitro and in vivo studies. In the present study was modifying effect of SRA on 7,12-dimethylbenz(a)anthracene (DMBA) induced cell surface glycoconjugates (GCs) abnormalities in the plasma and buccal mucosa of golden Syrian hamster buccal pouch carcinogenesis (HBPCs). Topical application of DMBA three times a week for 10 weeks on the buccal pouches of the hamsters resulted in well developed squamous cell carcinoma. GCs status was assessed biochemically, histological and immunoexpression pattern of cytokeratin (CK) in the buccal mucosa of the DMBA treated hamsters. Elevated levels of GCs and CK expression were observed in DMBA alone treated hamsters. Oral pre-administration of SRA (50 mg/kg bw) positively modulates the GCs levels and CK expressions to near normal. The present findings suggested that SRA can protect cell surface GCs and CK expression during DMBA induced HBPCs.
1. Introduction
Head and neck cancer is one of the major health problem globally, accounting for 247,000 new cases and 145,000 deaths annually GLOBACON 2012, with an average five years [[1], [2], [3], [4]]. In comparison with the global populations, where oral cancer represents only about 3% of malignancies, it accounts for over 30–40% of all cancers in India [5]. A common etiological factor like tobacco chewing, tobacco related products and drinking excessive alcohol are the most common factors for the development of oral cancer [6]. Tobacco smoking is an essential cause of human oral squamous cell carcinoma (OSCC). Tobacco smoke contains manifold carcinogens comprising polycyclic aromatic hydrocarbons and their derivatives [7]. DMBA, a polycyclic aromatic hydrocarbon, is an immunosuppressor as well as a potent organ-specific carcinogen [8]. Recently, studies have focused on the immunotoxicity of DMBA given to experimental animals to induce tumors in oral cancer [9,10]. DMBA is metabolically activated by cytochrome p450 monoxygenases to form electrophilic metabolite, diol epoxide, which binds covalently to adenine residues of DNA, forming DNA adducts that may eventually culminate in malignant transformation [11].
Recently, advanced therapeutic treatment of OSCC including surgical excision and radiation therapy has limited efficacy and results in an adverse systemic method for treating OSCC [12]. The occurrence of oral cancer is effectively reduced by the use of non-toxic chemopreventive agents that offers a believable approach. Chemoprevention refers to the organization of natural agents to prevent initiation, promotion, and progression events of carcinogenesis [13]. High consumption of novel natural products from plants, fruit and vegetables has long been used as a home remedy by the medical practitioners.
SRA is a major benzoic acid, derived from edible plants such as fruits and vegetables [14], and also used as a traditional Ayurvedic Indian medicine to treat diabetes. Several in vitro and in vivo investigations documented its beneficial role in various cancers and non communicable diseases [15,16]. SRA has multiple pharmacological properties such as antioxidant, anti-lipid peroxidative, anti-inflammatory, immunomodulatory, anti-endotoxic, anti-mitogenic and anti-cancer effects [[17], [18], [19], [20], [21],14]. SRA has potent anti-proliferative and hepatoprotective properties in human colorectal and breast cancer by scavenging their reactive oxygen species (ROS) [22,20,23]. In our previous dose dependent study, SRA completely inhibited the formation of exophytic tumor formation in DMBA induced HBPCs [24]. Velu et al. [25] reported that SRA has strong chemopreventive agents due to its modulating effect on detoxification metabolizing enzymes in DMBA-induced HBPCs.
Glycoproteins (GPs), a family of complex proteins in mammals, are the vital components on the cell surface and their glycan moieties exhibit diverse functions, from non-specific roles in protein structure and stability to specific ones in signal recognition [26]. Cell surface GPs play a key role in the neoplastic process and serve as an effective indicator of cancer progression. Cell surface GCs may contribute to play vital roles in various pathological actions such as cell-cell recognition, cell adhesion, antigenicity, inflammation, invasiveness and neoplastic transformation characteristics. Carbohydrate moieties of glycoprotein have also been implicated in the transport of metabolites across cell membranes and show a direct relationship between GCs and tumorigenesis [27].
CK is the epithelial specific intermediate filament proteins. CK is directly associated with the epithelial tumors of stratified buccal cell origin [28]. These are very sensitive marker in frequent molecular, biological, clinical and pathological studies [29]. IFs are necessary for intracellular components, underlying distinct properties and segregation stages in outer layer organs [30].
Since, no efficient studies exist on the effect of the SRA on GPs components in DMBA induced HBPCs. The aim of the current study to inspect the membrane stabilizing consequence of the SRA on cell surface GPs, CK and protein expression against DMBA induced OSCC in Syrian hamsters.
2. Materials and methods
2.1. Chemicals
DMBA (Fig. 1A) and SRA (Fig. 1B) were obtained from Sigma Aldrich Chemicals Pvt. Ltd (Bangalore, Karnataka, India). Primary and secondary antibodies against CK were supplied by Santa Cruz Biotechnology, USA. All other chemicals used in the study were of analytical grade, purchased by HIMEDIA Laboratories Pvt. Ltd., Mumbai, India.
Fig. 1.
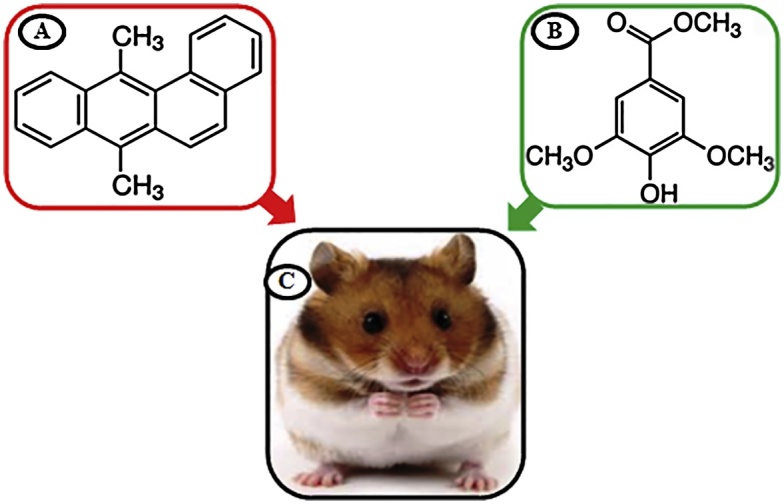
A) Structure of DMBA, B) Structure of SRA, C) Mesocricetus auratus.
2.2. Animals
Male golden Syrian hamsters (Mesocricetus auratus) (Fig. 1C), 8–10 weeks old, weighing 80–120 g, were purchased from the NIN, Hyderabad, India and maintained in the central animal house (CAH), Rajah Muthaiah Medical college and hospital, Annamalai University, India. They were housed four or five in a clean propylene cage and were provided daily standard pellet diet and water ad libitum. The hamsters were maintained, most commonly in controlled conditions of temperature (27 ± 2 °C) and humidity (55 ± 5%) with a 12 h light/dark cycle. The local institutional animal care and use committee (IACUC) (Reg No: 160/1999/CPCSEA) of Annamalai University approved the experimental guide (Proposal No: 1113, Dated: 16.04.2015).
2.3. Preparation of SRA
SRA (50 mg/kg bw) dissolved in 0.9% saline and given three times a week on days alternate to DMBA application, starting a week before the disclosure to the carcinogen and sustained during the experimental period (14 weeks).
2.4. Preparation of DMBA
OSCC was induced in hamster left buccal mucosa a No: 4 paint brush by topical application of DMBA (0.5% /kg bw) in liquid paraffin a thrice weekly for 10 weeks [31].
2.5. Experimental design
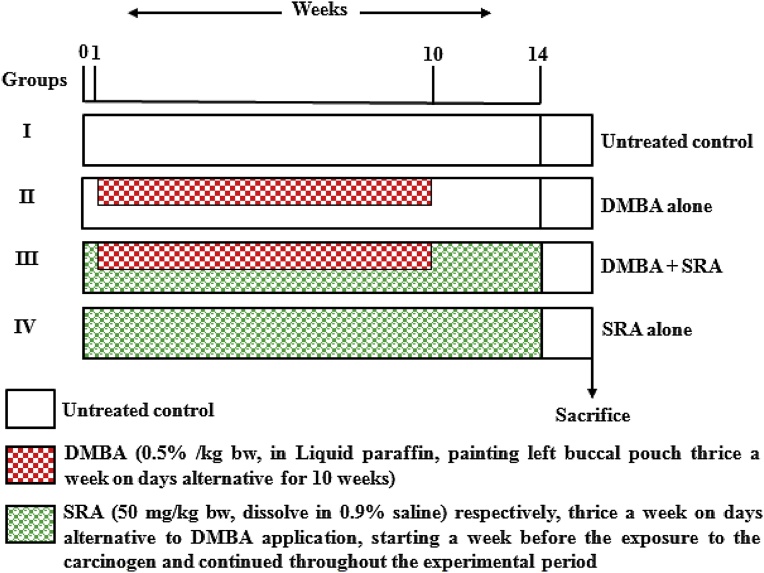
A total of forty hamsters were randomly assigned to four groups (n = 10 in each group). As shown in Fig. 2 the schematic protocol for optimum dose study. The experiment was terminated at the end of 14th week and all of the hamsters were sacrificed by cervical decapitation. Before the hamsters were killed, the left buccal mucosa was grossly inspected to evaluate premalignant lesions or tumor enlargement and photographed. The biochemical studies were carried out in the plasma and buccal pouch of untreated control and experimental hamsters in each group.
Fig. 2.
Schematic diagram for experimental design.
2.6. Tumor analysis
Total number of tumors present in all hamster cheek pouch epithelium were examined microscopically, when the hamsters were euthanized and the diameter of each tumor size was measured with a vernier caliper. The tumor volume calculations were measured using the formula, where D1, D2 and D3 are the three diameters (mm3) of the tumor were calculated by the methods of [32] respectively The tumor burden was determined by multiplying the tumor volume and number of tumors in each group.
2.7. Biochemical analysis
2.7.1. Sample collection
Biochemical samples were contacted on the blood and oral mucosal tissues of untreated control and experimental hamsters. Plasma was separated by centrifugation at 1000 × g for 15 min these arrangements were used for biochemical (GPs) estimations. Oral buccal tissues were washed with saline and homogenized using the Tris-HCl buffer in an all-glass Homogenizer with a Teflon pestle and used for biochemical (GCs) analysis.
The precipitate was obtained after treating the plasma with ethanol were used for the evaluation of GCs. The defatted tissues obtained after treating buccal mucosa tissues with methanol and chloroform were used for the estimation of GPs. The GPs in plasma and buccal mucosa tissues were expected by the methods of [[33], [34], [35], [36]] respectively. Proteins are estimated by the method of [37].
2.8. Histological studies
Histological investigations were performed on oral tissues of the untreated control and experimental hamsters. Tissues were fixed in 10% formalin and routinely processed and embedded in paraffin; 2–3 μm sections were used for histological Periodic Acid-Schiff (PAS) staining and immunohistochemistry (IHC) analysis. For detection of GCs, the tissue sections of buccal mucosa were immersed in a solution of 0.1% PAS staining for 15 min, at 50 °C. The slides were washed with running tap water and wrapped up in Schiff’s reagent for 40 min and the sections again washed for 10 min, counterstained with hematoxylin, dehydrated in graded ethanol, blank in xylene and mounted on resinous medium. Histological changes in the oral tissues of keratosis, hyperplasia, dysplasia and OSCC were observed under a light microscope.
2.9. Immunohistochemistry of buccal mucosa tissues
The oral mucosa tissue section fixed in formalin and fixed in paraffin. Therefore more incubated universal proteinaceous block to non-specific required sites and incubated with the primary antibody (CK) were used for IHC staining of proteins. The bound primary antibody was detected by incubation with the secondary antibody conjugated with HRP and DAB substrate. When acceptable color intensity is reached, counter stained with hematoxylin and covered with a mounting medium. We also graded CK expression according to the number of positive tumor cells per section 100 counted cells. The % of positive cells was scored according to the method of [38] and the IHC staining cells were variables shown in Table 3. A final arrangement was obtained for each score by using a phase contrast microscope (Nickon eclips 100, Germany).
Table 3.
The intensity of staining for cytokeratin expression in the buccal pouch of untreated control and experimental hamsters in each group (n = 10).
| Groups/Treatment | Cytokeratin |
|||
|---|---|---|---|---|
| 0 | 1+ | 2+ | 3+ | |
| Untreated control | 0 | 9 | 1 | 0 |
| DMBA | 0 | 0 | 0 | 10 |
| DMBA + SRA (50 mg/kg bw) | 0 | 6 | 4 | 0 |
| SRA alone (50 mg/kg bw) | 0 | 9 | 1 | 0 |
Results are given as number of hamsters (n = 10). The percentages of positive cells were scored as: 3+ = strong staining, more than 50% of the cells were stained, 2+ = moderate staining, between 20 and 50% of the cells were stained, 1+ = weak staining between 1 and 20% of the cells were stained, 0 = negative, less than 1% of cell staining.
2.10. Statistical analysis
Statistical analysis was performed using the Statistical Package for Social Sciences 17 (SPSS, Inc., Chicago). The data are expressed as the mean ± standard deviation (SD). We used one-way analysis of variance (ANOVA) by using Duncan Multiple Range Test (DMRT) group comparisons a method were used to correlate the difference between the variables. The value was considered statistically if the P value was less than 0.05.
3. Results
3.1. Effect of SRA on tumor incidence, number, volume, burden and histopathological changes
Table 1, Table 2 shows the tumor incidence, number, volume, burden and histopathological observations of untreated control and experimental hamsters. We observed 100 percentage tumor formation with mean tumor volume (150.68 mm3), tumor burden (1657.76 mm3) and well differentiated OSCC in DMBA alone hamsters (group 2). Pre-administration of SRA (50 mg/kg bw) (group 3) to DMBA treated hamsters significantly (p < 0.05) suppressed the development of tumor and exhibited moderate keratosis and mild hyperplasia. Normal cellular architecture and no tumor were observed in untreated control hamsters (group 1) as well as SRA alone administered hamsters (group 4).
Table 1.
Tumor incidence, number, volume and burden of untreated control and experimental hamsters in each group (n = 10).
| Groups/ Treatment | Untreated control | DMBA | DMBA + SRA (50 mg/kg bw) |
SRA alone (50 mg/kg bw) |
|---|---|---|---|---|
| Tumor incidence | 0% | 100% | 0% | 0% |
| Total number of tumor/hamsters | 0 | 11 ± 0.84 | 0 | 0 |
| Total volume (mm3)/hamsters | 0 | 150.68 ± 11.48 | 0 | 0 |
| Tumor burden (mm3)/hamsters | 0 | 1657.76 ± 63.23 | 0 | 0 |
Values are expressed as mean ± SD for ten hamsters in each group. Values not sharing a common superscript letter differ significantly at p < 0.05.
Tumor volume was measured using the formula where D1, D2 and D3 are the three diameters (mm3) of the tumor. The tumor burden was calculated by multiplying the tumor volume and number of tumors per hamster. Values are expressed as mean ± SD for 10 hamsters in each group.
Table 2.
Histopathological changes in the buccal mucosa of untreated control and experimental hamsters in each group (n = 10).
| Groups/Treatment | Untreated control | DMBA | DMBA + SRA (50 mg/kg bw) |
SRA alone (50 mg/kg bw) |
|---|---|---|---|---|
| Keratosis | – | +++ | ++ | ++ |
| Hyperplasia | – | +++ | + | + |
| Dysplasia | – | +++ | – | – |
| SCC | – | +++ | – | – |
– = No change, + = Mild, ++ = Moderate, +++ = Severe.
3.2. Estimation of GCs
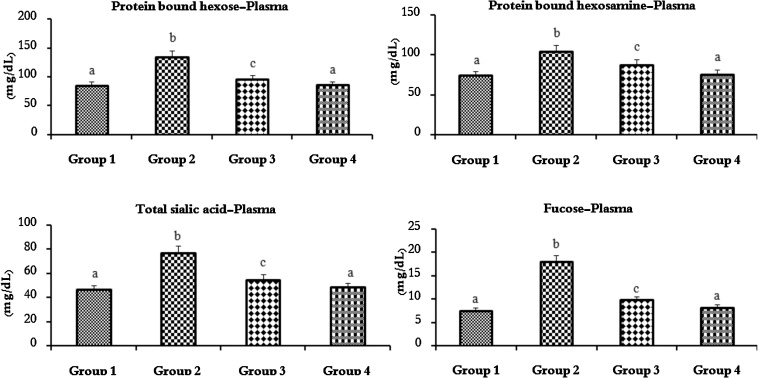
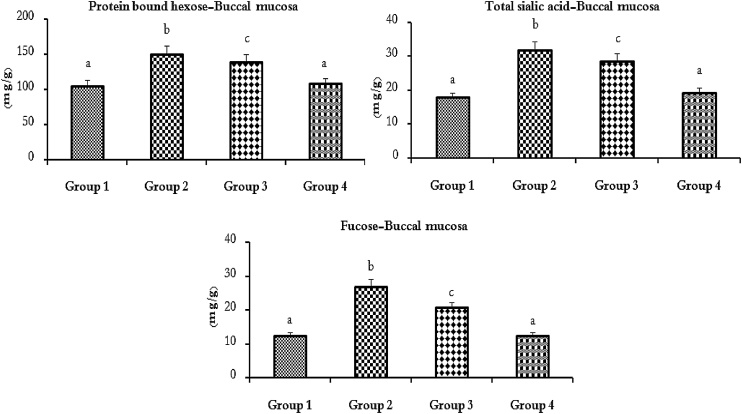
Fig. 3, Fig. 4 shows the levels of protein bound hexose, protein bound hexosamine, TSA and fucose in the plasma and buccal mucosa tissue in the untreated control and experimental hamsters in each group. The levels of GCs in the plasma were protein bound hexose, protein bound hexosamine, TSA and fucose and the buccal mucosa was protein bound hexose, TSA and fucose were significantly (p < 0.05) increased (100%) to DMBA (0.5% /kg bw) alone compared to untreated control hamsters. Oral pre-administration of SRA (50 mg/kg bw) to DMBA (0.5% /kg bw) painted hamsters significantly (p < 0.05) reduced the GCs levels (approximately 50–60%) near normal as compared to DMBA alone hamsters. No statistical significance was observed between untreated control and SRA alone treated hamsters.
Fig. 3.
Protein bound hexose, protein bound hexosamine, total sialic acid and fucose levels in the plasma of untreated control and experimental hamsters in each group (n = 10).
Values are expressed as mean ± SD for 10 experimental hamsters. Values that do not share a common superscript letter between groups differ significantly, at p < 0.05 (DMRT). Untreated control (group 1), DMBA alone (group 2), DMBA + SRA (group 3) and SRA alone (group 4).
Fig. 4.
Protein bound hexose, total sialic acid and fucose levels in the buccal mucosa of untreated control and experimental hamsters in each group (n = 10).
Values are expressed as mean ± SD for 10 experimental hamsters. Values that do not share a common superscript letter between groups differ significantly, at p < 0.05 (DMRT). Untreated control (group 1), DMBA alone (group 2), DMBA + SRA (group 3) and SRA alone (group 4).
3.3. Gross appearance and histopathological examination of mucosa tissues with PAS staining
The representative gross manifestation and photomicrographs of histopathological changes in the oral tissue of untreated control and experimental hamsters are shown in Fig. 5, Fig. 6. The GCs expression pattern was analyzed as evidenced by PAS staining in the buccal mucosa (Fig. 6). 100% tumor formation with significantly (p < 0.05) increased positive percentage PAS staining (increased the expression of GCs) were observed in DMBA alone (group 2) as compared to untreated control hamsters. Oral pre-administration of SRA (50 mg/kg bw) to DMBA painted hamsters (group 3) significantly (p < 0.05) reduced the percentage of PAS positive cells (decreased the expression of GCs) as compared to DMBA alone. The GCs expression pattern was normal in SRA alone treated (group 4) and untreated control (group 1). These results revealed a significant positive correlation with biochemical glycoconjugate analysis.
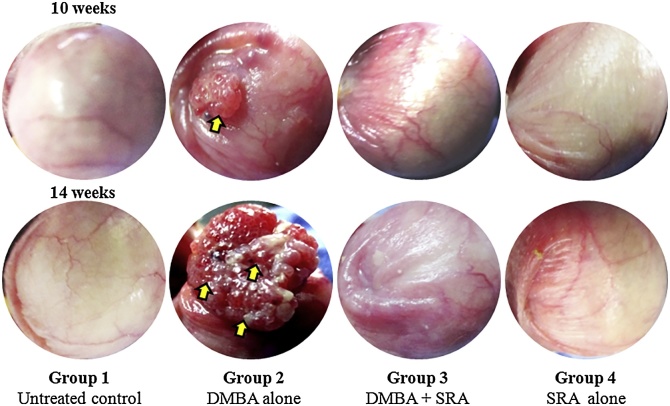
Fig. 5.
Photomicrograph showing the gross appearance of oral squamous cell carcinomas on 10th and 14th week indicated by arrow (). Gross appearance of oral tissue of untreated control and experimental hamsters in each group (40x).
Group 2 exophytic and well defined tumor mass in the HBP painted with DMBA alone. Untreated control (group 1), DMBA + SRA (group 3) and SRA alone (group 4) treated hamsters revealed that the normal appearance of the buccal mucosa. The images show sections of the oral buccal mucosa stained with PAS embracing the buccal mucosa area (40x).
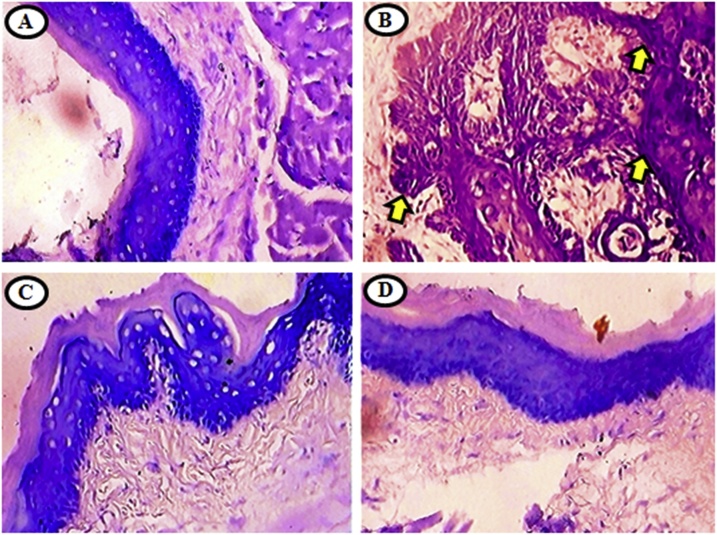
Fig. 6.
Histological analysis of GCs by PAS in the buccal mucosa of untreated control and experimental hamsters in each group (40x).
A and D) shows GCs expression pattern was normal. B) shows well differentiated OSCC with keratin pearls shown over expression of GCs. C) shows lowered expressions and mild dysplasia with hyperkeratosis of GCs.
3.4. Effects of IHC expression in CK of buccal tissues
Effect of SRA on CK expression in the buccal pouch mucosa of untreated control and experimental hamsters in each group and IHC scores of positively stained cells were shown in Table 3 and Fig. 7. The expression of CK was significantly (p < 0.05) higher in DMBA alone painted hamsters as compared to untreated control hamsters. Pre-administration of SRA (50 mg/kg bw) to DMBA treated hamsters showed significantly (p < 0.05) reduce the expression of CK when compared to DMBA alone painted hamsters. Normal CK expression was observed in untreated control and SRA alone treated hamsters.
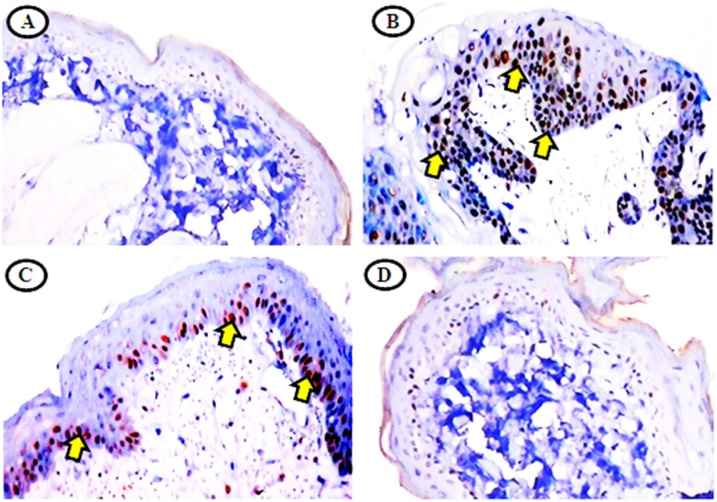
Fig. 7.
IHC expression of CK in untreated control and experimental hamsters in each group (40x).
A and D) microphotography showing normal levels of CK expression in untreated control and SRA alone treated hamsters. B) showing over expression of CK in DMBA alone treated hamsters. C) showing mild to restrained CK expression in DMBA + SRA treated hamsters.
4. Discussion
Oral cancer has become a significant health care problem clinically. However, promising therapeutic and preventive strategy for the different stages of the oral carcinogenesis (OCs) process could be important for management of patients. The HBP has been used since 1954 as a model for oral cavity cancer [39] and studies revealed the inhibition of oral cancer by numerous phytochemical agents. A chemopreventive agent has been shown to reduce growth of tumors in the HBP when administered before, during and after chemical carcinogen [40]. Natural synthetic compounds are particularly helpful for chemoprevention of oral cancer in HBP.
Kumar et al. [41] have been reported that SRA protects hypersensitive rats at different doses (25, 50 and 100 mg/kg bw) SRA at a dose 50 mg/kg bw, exerts optimum protection, reducing oxidative stress and also retaining the bioavailability of nitrous oxide in the cardiovascular system. In our previous dose dependent study, SRA has chemopreventive potential in DMBA induced HBPCs, hence among the different doses (25, 50 and 100 mg/kg bw), the effect of SRA at the doses of 50 and 100 mg/kg bw, was being pronounced effects in DMBA induced hamsters. Further, in all our experimental design we used medium dose (50 mg/kg bw) to evaluate the effects on DMBA induced cell surface GCs and CK expressions [24].
In our present study, topical application of DMBA to the HBP for 10 weeks induced OSCC with a very large tumor burden 1657.76 ± 63.23 mm3 associated with aberrant expression of CK (80%) is a 3+ = strong staining. Oral administration of SRA at a dose of 50 mg/kg bw, delayed the growth of tumors in the oral tissue of DMBA-treated hamsters. Our results suggested that SRA might be suppressed abnormal growth occurring during DMBA-induced OCs as evidenced by prevention of tumor formation in the buccal mucosa of DMBA treated hamsters. Previously we reported that SRA prevented the formation of excess ROS in hamsters treated with DMBA as evidenced by the increased activities of antioxidants. Due to orthodiphenol content in SRA, which is playing a radical scavenging effect on HBPCs [24]. Orabi et al. [17] demonstrated that SRA possesses anti-mutagenic activity against human malignant melanoma cells. Yan et al. [42] reported that SA inhibits reactive oxygen species and inflammatory markers in acute ethanolic induced-hepatotoxicity in mice. SRA exhibits a number of mechanisms involved in anti-proliferative and apoptosis induction in human breast cancer cells [43].
GCs were secreted by cancer cells are a most potential source to assess the progression of cancer biomarkers. At early stages, measurement of these biomarkers could be useful for diagnosis and prognosis of all cancers. Shah et al. [44] suggested that the tissue and circulatory GPs levels of analysis are used OCs. Stowell et al. [45] demonstrated that modifications of oligosaccharide moieties on the surface of cancer cell GPs are associated with invasion and metastasis of cancers.
Sialic acid is very important to determine the surface properties of cells and has been implicated in cellular invasiveness, adhesiveness and immunogenicity [46]. Previous studies in our laboratory demonstrated that malignant cells have been further sialic acid in their cell membrane than in normal cells and elevation of TSA in the plasma were found to reflect tumor burden, and correlated well with several stages of cancers including oral cancer [47]. Our results are in line with these studies. Fucose plays an important function in several non-communicable diseases including oral cancer its invasion and metastasis of cancer cells [48]. Raval et al. [49] reported that exposure of carcinogen improved sialyl transferase movement and has been responsible for amplified expression of cell surface GCs during DMBA induced OCs. The results are corroborated by these observations. Dualistic increases in the plasma and buccal tissue sialic acid content in tumor bearing hamsters reported [50]. An increased fucose substance in the oral tumor and plasma is probably due to increased proceeds of malignant cells with subsequent leakage into distribution [7]. Elevated levels of fucose, a terminal pentose sugar of glycoprotein chain, have been reported in the various types of malignancies [51,52]. It has been suggested prominent levels of TSA and fucose in the cell surface may facilitate tumor metastasis. Improved fucosylation in the cancer cell surface might contribute to decreased adhesion and uncontrolled growth [53].
Increased glycoprotein levels in cancer cell are associated with cell proliferation rather than cell destruction. Due to abnormal polymerization or the turnover of glycoprotein content on the surface of the cancer cell results in leakage/shedding of soluble biomolecules into the plasma [54,45]. Aberrant glycosylation responsible for the hyper disordered proliferation observed in oral dysplastic leukoplakias and carcinomas that are either absent of normal glycosyltransferases or the activation of new tumor related enzymes [55]. Profound studies have shown that atypical glycosylation and degradation of cell surface carbohydrates have been shown in OCs [56]. Increased levels of protein bound hexose, hexosamine and TSA and fucose have been reported in experimental HBPCs [27]. Muthukumaran et al. [57] revealed that oral administration of SRA brought the levels of hexose, hexosamine, TSA and fucose to near normal level in streptozotocin induced diabetic rats and also it inhibited the synthesis of GPs. Elevated sialyl and glycosyltransferase activity could be responsible for over expression of cell surface GCs in malignant tumor [58]. Oral administration of SRA to hamsters treated with DMBA significantly inhibit the levels of plasma and oral tissue GCs. SRA inhibited the formation of GPs during neoplastic transformation through altered the activities of glycosylation enzymes (glycosyltransferases and glycosidases).
Augmented glycosylation of membrane bio-molecules causes accumulation of GCs in the tumor tissue and is related to the degree of keratosis [59]. Increased staining intensity (pink color) of buccal mucosal cells with PAS positive reaction is probably due to GCs accumulation in tumor cells. Administration of SRA to DMBA induced hamsters, delayed the keratinization of epithelial tissue and prevented cell proliferation. Histological assessment of the GCs expression model in the experimental hamsters interrelated with the biochemical findings. Thus, SRA protects cell surface abnormality in DMBA-induced OCs.
Profound studies shown that the aberrant expression of CK as a main family of intermediary filaments (IFs) might add supplementary prognostic significance in the tumorigenesis [60]. Detection of GCs and CK expression is helping to assess the cancer patients with malignant neoplasm [61]. Several studies reported that abnormal expression in the CK has been experimenting in a wide range of epithelial carcinomas [62,63]. CK is a useful proliferation marker and a major component in IFs of structural proteins. Thus, the purpose of the present study was to find the immunoexpression pattern of the CK in buccal tissues as a useful tool for objectively distinguishing dysplastic buccal pouch epithelium. Several reports documented that elevated expression of CK is associated with neoplastic malignant transformation [62,64]. Babu et al. [65] demonstrated that hesperetin significantly inhibit the status of the CK expression to the near normal range to DMBA treated hamsters. The immunoexpression was elevated in CK is used for confirming the presence of dysplastic changes in DMBA alone painted hamsters. The positivity of staining was always strong in a high percentage of cells in the DMBA alone treated hamsters. The IHC pattern is clearly differentiated from normal buccal mucosa, in which the whole cells are weakly positive in SRA treated hamsters. In this present study, SRA has chemopreventive efficacy and protected the abnormalities on the cell surface GCs and near normal the expression of CK in DMBA induced HBPCs (Fig. 8).
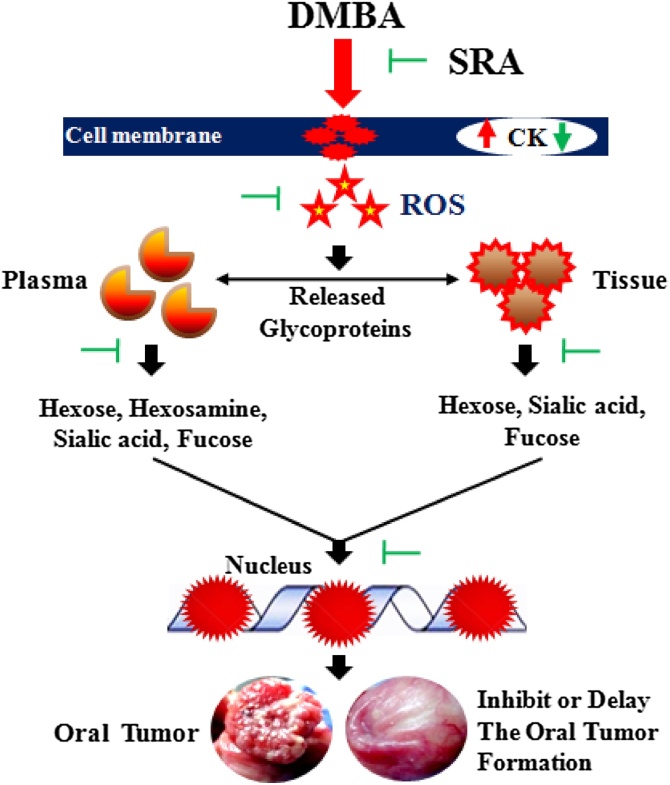
Fig. 8.
Putative action of SRA in DMBA induced cell surface GCs and CK changes in HBPCs.
Pre-administration of SRA (50 mg/kg bw) to DMBA treated suppressed the development of tumor and exhibited moderate keratosis, mild hyperplasia and the expression of CK. SRA inhibited the formation of GPs during neoplastic transformation through altered the activities of glycosylation. Administration of SRA to DMBA induced hamsters, delayed the keratinization of epithelial tissue and prevented cell proliferation. Histological assessment of the GCs expression model in the experimental hamsters interrelated with the biochemical findings. Thus, SRA protects cell surface abnormality in DMBA-induced HBPCs.
5. Conclusion
In summary, oral administration of SRA to DMBA treated hamsters significantly repressed the abnormalities seen on cell surface GCs in the oral tissues and circulation during OCs and restored the expression of CK, which indicates the membrane stabilizing effect of SRA during neoplastic alteration. The protective role of the SRA on cell surface GPs is probably due to its inhibitory role in the abnormal GPs separation and on regulating glycosyltransferase activity.
Conflict of interest
The authors declare that there are no conflicts of interest related to this work.
Ethical approval
All the animals were maintained in accordance with the guidelines of CPCSEA and the experimental design was permitted by the IACUC (Reg No: 160/1999/CPCSEA) of Annamalai University approved the experimental plan (Proposal No: 1113, Dated: 16.04.2015).
Transparency document
References
- 1.Ferlay J., Soerjomataram I., Dikshit R., Eser S., Mathers C., Rebelo M., Parkin D.M., Forman D., Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer. 2012;136(5):E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.Molinolo A.A., Amornphimoltham P., Squarize C.H., Castilho R.M., Patel V., Gutkind J.S. Dysregulated molecular networks in head and neck carcinogenesis. Oral Oncol. 2009;45(4–5):324–334. doi: 10.1016/j.oraloncology.2008.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang X., Junior C.R., Liu M., Li F., D’Silva N.J., Kirkwood K.L. Oral squamous carcinoma cells secrete RANKL directly supporting osteolytic bone loss. Oral Oncol. 2013;49(2):119–128. doi: 10.1016/j.oraloncology.2012.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chi A.C., Day T.A., Neville B.W. Oral cavity and oropharyngeal squamous cell carcinoma – an update. CA Cancer J. Clin. 2015;65:401–421. doi: 10.3322/caac.21293. [DOI] [PubMed] [Google Scholar]
- 5.Dangi J., Kinnunen T.H., Zavras A.I. Challenges in global improvement of oral cancer outcomes: findings from rural Northern India. Tob. Induc. Dis. 2012;10:5. doi: 10.1186/1617-9625-10-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang T.T., Huang J.S., Wang Y.Y., Chen K.C., Wong T.Y., Chen Y.C., Wu C.W., Chan L.P., Lin Y.C., Kao Y.H., Nioka S., Yuan S.F., Chung P.C. Novel quantitative analysis of autofluorescence images for oral cancer screening. Oral Oncol. 2017;68:20–26. doi: 10.1016/j.oraloncology.2017.03.003. [DOI] [PubMed] [Google Scholar]
- 7.Chen K.M., Sacks P.G., Spratt T.E., Lin J.M., Boyiri T., Schwartz J., Richie J.P., Calcagnotto A., Das A., Bortner J., Zhao Z., Amin S., Guttenplan J., El-Bayoumy K. Modulations of benzo[a]pyrene-induced DNA adduct, cyclin D1 and PCNA in oral tissue by 1,4-phenylenebis(methylene)selenocyanate. Biochem. Biophys. Res. Commun. 2009;383(1):151–155. doi: 10.1016/j.bbrc.2009.03.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miyata M., Furukawa M., Takahashi K., Gonzalez F.J., Yamazoe Y. Mechanism of 7,12-dimethylbenz[a]anthracene-induced immunotoxicity: role of metabolic activation at the target organ. Jpn. J. Pharmacol. 2001;86(3):302–309. doi: 10.1254/jjp.86.302. [DOI] [PubMed] [Google Scholar]
- 9.Chen X., Zhang X., Lu Y., Shim J.Y., Sang S., Sun Z., Chen X. Chemoprevention of 7,12-dimethylbenz[a]anthracene (DMBA)-induced Hamster Cheek Pouch Carcinogenesis by a 5-Lipoxygenase Inhibitor. Nutr. Cancer. 2012;64(8):1211–1218. doi: 10.1080/01635581.2012.718032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dean J.H., Ward E.C., Murray M.J., Lauer L.D., House R.V., Stillman W., Hamilton T.A., Adams D.O. Immunosuppression following 7,12-dimethylbenz[a]anthracene exposure in B6C3F1 mice – II. Altered cell-mediated immunity and tumor resistance. Int. Immunopharmacol. 1986;8(2):189–198. doi: 10.1016/0192-0561(86)90058-5. https://www.sciencedirect.com/science/article/pii/0192056186900585?via%3Dihub [DOI] [PubMed] [Google Scholar]
- 11.Christou M., Moore C.J., Gould M.N., Jefcoate C.R. Induction of mammary cytochromes P-450: an essential first step in the metabolism of 7,12-dimethylbenz[a]anthracene by rat mammary epithelial cells. Carcinogenesis. 1987;8(1):73–80. doi: 10.1093/carcin/8.1.73. [DOI] [PubMed] [Google Scholar]
- 12.Fung C., Grandis J.R. Emerging drugs to treat squamous cell carcinomas of the head and neck. Expert Opin. Emerg. Drugs. 2010;15(3):355–373. doi: 10.1517/14728214.2010.497754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Russo G.L. Ins and outs of dietary phytochemicals in cancer chemoprevention. Biochem. Pharmacol. 2007;74(4):533–544. doi: 10.1016/j.bcp.2007.02.014. [DOI] [PubMed] [Google Scholar]
- 14.Karthik G., Angappan M., VijayaKumar A., Natarajapillai S. Syringic acid exerts antiangiogenic activity by downregulation of VEGF in zebrafish embryos. Biochem. Pharmacol. 2007;4(2):203–208. [Google Scholar]
- 15.Kumar S., Pandey A.K. Chemistry and biological activities of flavonoids: an overview. Sci. World J. 2013;16 doi: 10.1155/2013/162750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boutayeb A., Boutayeb S. The burden of non communicable diseases in developing countries. Int. J. Equity Health. 2005;4:2. doi: 10.1186/1475-9276-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Orabi K.Y., Abaza M.S., Sayed K.A., Elnagar A.Y., Al-Attiyah R., Guleri R.P. Selective growth inhibition of human malignant melanoma cells by syringic acid-derived proteasome inhibitors. Cancer Cell Int. 2013;13:1. doi: 10.1186/1475-2867-13-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zambonin L., Caliceti C., Vieceli Dalla Sega F., Fiorentini D., Hrelia S., Landi L., Prata C. Dietary phenolic acids act as effective antioxidants in membrane models and in cultured cells, exhibiting proapoptotic effects in leukaemia cells. Oxid. Med. Cell. Longev. 2012:839298. doi: 10.1155/2012/839298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ham J.R., Lee H.I., Choi R.Y., Sim M.O., Seo K.I., Lee M.K. Anti-steatotic and anti-inflammatory roles of syringic acid in high-fat diet-induced obese mice. J. Funct. Foods. 2016;2016(7):689–697. doi: 10.1039/c5fo01329a. [DOI] [PubMed] [Google Scholar]
- 20.Itoh A., Isoda K., Kondoh M., Kawase M., Kobayashi M., Tamesada M., Yagi K. Hepatoprotective effect of syringic acid and vanillic acid on CCl4-induced liver injury. Biol. Pharm. Bull. 2010;33(6):983–987. doi: 10.1248/bpb.33.983. [DOI] [PubMed] [Google Scholar]
- 21.Liu Y., Fang J., Lei T., Wang W., Lin A. Anti-endotoxic effects of syringic acid of Radix isatidis. J. Huazhong Univ. Sci. Technol. 2003;23(2):206–208. doi: 10.1007/BF02859960. [DOI] [PubMed] [Google Scholar]
- 22.Siew Hon N.G. Vol. 2013. University Saskatchewan; 2011. https://ecommons.usask.ca/handle/10388/ETD-2011-09-163 (Master Thesis. Characterization Colon Cancer Cell Culture Based Screening Assay to Study Effects of Phenolic Acids). [Google Scholar]
- 23.Ramachandran V., Raja B. Protective effects of syringic acid against acetaminophen induced hepatic damage in albino rats. J. Basic Clin. Physiol. Pharmacol. 2010;21(4):369–386. doi: 10.1515/jbcpp.2010.21.4.369. [DOI] [PubMed] [Google Scholar]
- 24.Velu P., Vinothkumar V., Babukumar S., Ramachandhiran D. Chemopreventive effect of syringic acid on 7,12-dimethylbenz(a)anthracene induced hamster buccal pouch carcinogenesis. Toxicol. Mech. Methods. 2017;27(8):631–640. doi: 10.1080/15376516.2017.1349227. [DOI] [PubMed] [Google Scholar]
- 25.Velu P., Vinothkumar V., Babukumar S., Ramachandhiran D. Syringic acid, a polyphenol modulates detoxification agents on 7,12-dimethylbenz[a]-anthracene induced oral carcinogenesis in golden Syrian hamsters. Int. J. Adv. Sci. Res. 2017;2(6):90–95. https://www.allsciencejournal.com/search?keyword=syringic+acid [Google Scholar]
- 26.Martin A., Rambal C., Berger V., Perier S., Louisot P. Availability of specific sugars for glycoconjucate biosynthesis: a need for further investigations in man. Biochimie. 1998;80(1):75–86. doi: 10.1016/s0300-9084(98)80059-x. [DOI] [PubMed] [Google Scholar]
- 27.Thirunavukkarasu C., Sakthisekaran D. Influence of sodium selenite on glycoprotein contents in normal and N-nitrosodiethylamine initiated and phenobarbital promoted rat liver tumors. Pharmacol. Res. 2003;48(2):167–173. doi: 10.1016/s1043-6618(03)00104-x. [DOI] [PubMed] [Google Scholar]
- 28.Vander Velden L.A., Mahni J.J., Ramaekers F.C., Kuijpers W. Expression of intermediate filament proteins in benign lesions of the oral mucosa. Eur. Arch. Oto-Rhino-Laryngology. 1999;256(10):514–519. doi: 10.1007/s004050050202. https://www.ncbi.nlm.nih.gov/pubmed/10638360 [DOI] [PubMed] [Google Scholar]
- 29.Fillies T., Jogschies M., Kleinheinz J., Brandt B., Joos U., Buerger H. Cytokeratin alteration in oral leukoplakia and oral squamous cell carcinoma. Oncol. Rep. 2007;18(3):639–643. [PubMed] [Google Scholar]
- 30.Ogden G.R. Alcohol and oral cancer. Alcohol. 2005;35(3):169–173. doi: 10.1016/j.alcohol.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 31.Shklar G. Development of experimental oral carcinogenesis and its impact on current oral cancer research. J. Dent. Res. 1999;78(12):1768–1772. doi: 10.1177/00220345990780120101. [DOI] [PubMed] [Google Scholar]
- 32.Vinothkumar V., Manoharan S. Chemopreventive efficacy of geraniol against 7,12-dimethylbenz[a]anthracene-induced hamster buccal pouch carcinogenesis. Redox Rep. 2011;16(3):91–100. doi: 10.1179/174329211X13020951739839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Niebes P., Berson I. Determination of enzyme and degradation products of GAG metabolism in the serum of healthy and various subjects. Clinic. Chim. Acta. 1973;42(2):399–408. [PubMed] [Google Scholar]
- 34.Wagner W.D. A more sensitive assay discriminating galactosamine and glucosamine in mixture. Anal. Biochem. 1979;94(2):394–396. doi: 10.1016/0003-2697(79)90379-8. [DOI] [PubMed] [Google Scholar]
- 35.Warren L. Thiobarbituric acid and assay of sialic acid. J. Biol. Chem. 1959;234(8):1971–1975. https://www.jbc.org/content/234/8/1971.long [PubMed] [Google Scholar]
- 36.Dische L., Shettles L.B. Specific color reactions of methyl pentoses and spectrophotometric micromethod for their determination. J. Biol. Chem. 1948;175:595–603. https://www.jbc.org/content/175/2/595.full.pdf [PubMed] [Google Scholar]
- 37.Lowry O.H., Rosebrough N.J., Farr A.L., Randall R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951;193(1):265–275. https://www.jbc.org/content/193/1/265.long [PubMed] [Google Scholar]
- 38.Lyzogubov V., Khozhaenko Y., Usenko V., Antonjuk S., Ovcharenko G., Tikhonkova I., Filonenko V. Immunohistochemical analysis of Ki-67, PCNA and S6K1/2 expression in human breast cancer. Exp. Oncol. 2005;27(2):141–144. https://www.ncbi.nlm.nih.gov/pubmed/15995633 [PubMed] [Google Scholar]
- 39.Salley J.J. Experimental carcinogenesis in the cheek pouch of the Syrian hamster. J. Dent. Res. 1954;33(2):253–262. doi: 10.1177/00220345540330021201. [DOI] [PubMed] [Google Scholar]
- 40.Tanaka T. Chemoprevention of oral carcinogenesis. Eur. J. Cancer, B, Oral Oncol. 1995;31(1):3–15. doi: 10.1016/0964-1955(94)00026-z. [DOI] [PubMed] [Google Scholar]
- 41.Kumar S., Prahalathan P., Raja B. Syringic acid ameliorates (L)-NAME-induced hypertension by reducing oxidative stress. Naunyn Schmiedebergs Arch. Pharmacol. 2012;385(12):1175–1184. doi: 10.1007/s00210-012-0802-7. [DOI] [PubMed] [Google Scholar]
- 42.Yan S.L., Wang Z.H., Yen H.F., Lee Y.J., Yin M.C. Reversal of ethanol-induced hepatotoxicity by cinnamic and syringic acids in mice. Food Chem. Toxicol. 2016;98:119–126. doi: 10.1016/j.fct.2016.10.025. [DOI] [PubMed] [Google Scholar]
- 43.Kampa M., Alexaki V.I., Notas G., Nifli A.P., Nistikaki A., Hatzoglou A., Bakogeorgou E., Kouimtzoglou E., Blekas G., Boskou D., Gravanis A., Castanas E. Antiproliferative and apoptotic effects of selective phenolic acids on T47D human breast cancer cells: potential mechanisms of action. Breast Cancer Res. 2004;6(2):R63–R74. doi: 10.1186/bcr752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shah F.D., Begum R., Vajaria B.N., Patel K.R., Patel J.B., Shukla S.N., Patel P.S. A review on salivary genomics and proteomics biomarkers in oral cancer. Indian J. Clin. Biochem. 2011;26(4):326–334. doi: 10.1007/s12291-011-0149-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stowell S.R., Ju T., Cummings R.D. Protein glycosylation in cancer. Annu. Rev. Pathol. Mech. Dis. 2015;10(1):473–510. doi: 10.1146/annurev-pathol-012414-040438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chaudhari V., Pradeep G.L., Prakash N., Mahajan A.M. Estimation of salivary sialic acid in oral premalignancy and oral squamous cell carcinoma. Contemp. Clin. Dent. 2016;7(4):451–456. doi: 10.4103/0976-237X.194108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vinothkumar V., Manoharan S., Palanimuthu D., Rajasekaran D., Srinivasan R., Afaq Wani S. Geraniol protects cell surface glycoconjugates abnormalities during 7, 12- dimethylbenz (a) anthracene induced oral carcinogenesis. J. Cell Tissue Res. 2011;11(2):2759–2764. [Google Scholar]
- 48.Singh A.K., Gopu K. Synthesis and antioxidant properties of novel α-tocopherol glycoconjugates. Tetrahedron Lett. 2009;51(8):1180–1184. [Google Scholar]
- 49.Raval G.N., Parekh L.J., Patel D.D., Jha F.P., Sainger R.N., Patel P.S. Clinical usefulness of alterations in sialic acid, sialyl transferase and sialoproteins in breast cancer. Indian J. Clin. Biochem. 2004;19(2):60–71. doi: 10.1007/BF02894259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zachariah B., Basu D. Surface carbohydrates in cell biology. Indian J. Biochem. Biophys. 1993;30:422–425. [PubMed] [Google Scholar]
- 51.Fernandez-Rodriguez J., Paez-de-la-cadena M., Martinez-zorzano V.S. Fucose level in serum and in tumor of colorectal adenocarcinoma patients. Cancer Lett. 1997;121(2):147–153. doi: 10.1016/s0304-3835(97)00343-1. [DOI] [PubMed] [Google Scholar]
- 52.Sanjay P.R., Hallikeri K., Shivashankara A.R. Evaluation of salivary sialic acid, total protein, and total sugar in oral cancer: a preliminary report. Indian J. Dent. Res. 2008;19(4):288–291. doi: 10.4103/0970-9290.44529. [DOI] [PubMed] [Google Scholar]
- 53.Tomsik P., Soukup T., Cermakova E., Miscuda S., Niang M., Sucha L., Rezacova M. L-rhamnose and L-fucose suppress cancer growth in mice. Cent. Eur. J. Biol. 2011;6(1):1–9. [Google Scholar]
- 54.Hauselmann I., Borsig L. Altered tumor-cell glycosylation promotes metastasis. Front. Oncol. 2014;4:28. doi: 10.3389/fonc.2014.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gron B., Andersson A., Dabelsteen E. Blood-grouprelated carbohydrates are expressed in organotypic cultures of human skin and oral mucosa. APMIS. 1999;107(7-12):779–790. doi: 10.1111/j.1699-0463.1999.tb01473.x. [DOI] [PubMed] [Google Scholar]
- 56.Banerjee A.G., Bhattacharyya I., Lydiatt W.M., Vishwanatha J.K. Aberrant expression and localization of decorin in human oral dysplasia and squamous cell carcinoma. Cancer Res. 2003;63(22):7769–7776. https://cancerres.aacrjournals.org/content/63/22/7769.long [PubMed] [Google Scholar]
- 57.Muthukumaran J., Srinivasan S., Venkatesan R.S., Ramachandran V., Muruganathan U. Syringic acid, a novel natural phenolic acid, normalizes hyperglycemia with special reference to glycoprotein components in experimental diabetic rats. J. Acute Dis. 2013;2(4):304–309. [Google Scholar]
- 58.Sebzda T., Saleh Y., Gburek J., Warwas M., Andrzejak R., Siewinski M., Rudnicki J. Total and lipid-bound plasma sialic acid as diagnostic markers in colorectal cancer patients: correlation with cathepsin B expression in progression to Dukes stage. J. Exp. Ther. Oncol. 2006;5(3):223–229. https://www.ncbi.nlm.nih.gov/pubmed/16528972 [PubMed] [Google Scholar]
- 59.Robledo Y., Marigomez I., Angulo E., Cajaraville M.P. Glycosylation and sorting pathways of lysosomal enzymes in mussel digestive cells. Cell Tissue Res. 2006;324(2):319–333. doi: 10.1007/s00441-005-0125-9. [DOI] [PubMed] [Google Scholar]
- 60.Watanabe S., Ichikawa E., Takahashi H., Otsuka F. Changes of cytokeratin and involucrin expression in squamous cell carcinomas of the skin during progression to malignancy. Br. J. Dermatol. 1995;132(5):730–739. doi: 10.1111/j.1365-2133.1995.tb00718.x. [DOI] [PubMed] [Google Scholar]
- 61.Reis C.A., Osorio H., Silva L., Gomes C., David L. Alterations in glycosylation as biomarkers for cancer detection. J. Clin. Pathol. 2010;63(4):322–329. doi: 10.1136/jcp.2009.071035. [DOI] [PubMed] [Google Scholar]
- 62.Morgan P.R., Su L. Intermediate filaments in oral neoplasia. I. Oral cancer and epithelial dysplasia. Eur. J. Cancer, B, Oral Oncol. 1994;30B(3):160–166. doi: 10.1016/0964-1955(94)90085-x. https://www.ncbi.nlm.nih.gov/pubmed/7522710 [DOI] [PubMed] [Google Scholar]
- 63.Singh A., Kapur S., Chattopadhyay I., Purkayastha J., Sharma J., Mishra A., Hewitt S.M., Saxena S. Cytokeratin immunoexpression in esophageal squamous cell carcinoma of high-risk population in Northeast India. Appl. Immunohistochem. Mol. Morphol. 2009;17(5):419–424. doi: 10.1097/PAI.0b013e31819d3753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Layfield L.J., Emerson L., Crim J.R., Randall L. Squamous differentiation and cytokeratin expression in an osteosarcoma: a case report and review of the literature. Clin. Med. Insights Pathol. 2008;2008(1):55–59. doi: 10.4137/cpath.s582. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3160007/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Babukumar S., Vinothkumar V., Velu P., Ramachandhiran D. Hesperetin on cell surface glycoconjugates abnormalities and immunohistochemical staining with cytokeratin in 7,12 dimethylbenz(a)anthracene induced Hamster Buccal pouch carcinogenesis. Indian J. Clin. Biochem. 2017;33(4):438–444. doi: 10.1007/s12291-017-0704-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.