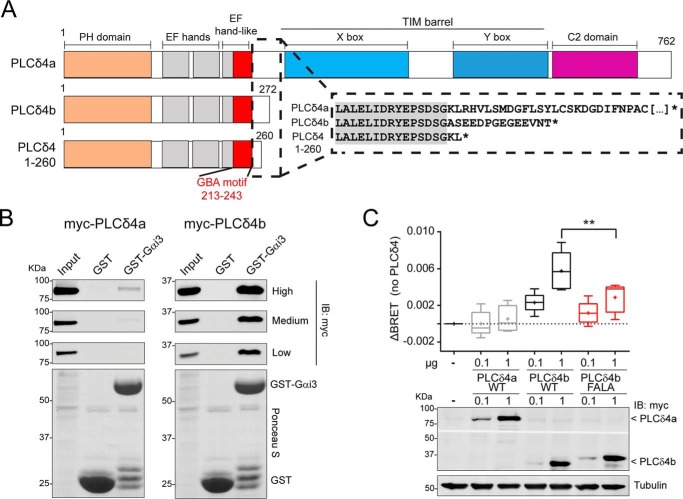
Figure 9.
PLCδ4b binds Gαi3 more efficiently than PLCδ4a and activates G-proteins in cells. A, bar diagrams of human PLCδ4 isoforms and constructs. PLCδ4a contains (from N to C terminus) a PH domain, two EF-hands, an EF-hand–like domain that overlaps with the GBA motif, a catalytic TIM barrel (with X- and Y-boxes), and a C2 domain. PLCδ4b and PLCδ4(1–260) are truncated before the catalytic TIM barrel. Inset, partial sequence alignment of PLCδ4a, PLCδ4b, and PLCδ4(1–260). The C-terminal sequence of PLCδ4b differs from PLCδ4a in the 14 aa preceding its stop codon, and the PLCδ4(1–260) construct used in previous experiments (Figs. 2–5) is very similar to PLCδ4b. B, Gαi3 binding of two PLCδ4 isoforms. Approximately 30 μg of purified GST-Gαi3 or GST immobilized on GSH-agarose beads were incubated with lysates of HEK293T cells expressing either myc-PLCδ4a (left) or myc-PLCδ4b (right) in the presence of GDP. Resin-bound proteins were eluted, separated by SDS-PAGE, and analyzed by Ponceau S-staining and immunoblotting (IB) with the indicated antibodies. Three different capture intensities (low, medium, and high) are shown for the Myc immunoblotting. Input = 2.5% of the total amount of lysate added in each binding reaction. One representative experiment of at least three is shown. C, PLCδ4b, but not PLCδ4a, activates G-protein signaling in cells via its GBA motif. HEK293T cells were transfected with the indicated amounts of plasmids for the expression of myc-PLCδ4a (WT) or myc-PLCδ4b (WT or FALA mutants) and all the required components of the BRET-based G-protein activity assay described in Fig. 8A (Gαi3, V-Gβγ, and GRK3ct-Nluc). Results from n = 5 are presented as the increase in BRET (ΔBRET) compared with cells not expressing PLCδ4 in a box-and-whisker plot (median with boxes of 25–75%), and error bars indicate the range. Cross-marks indicate the average). An immunoblot of a representative experiment is shown below the graph. **, p < 0.01 compared with the respective WT using the Student's t test.