Figure 1.
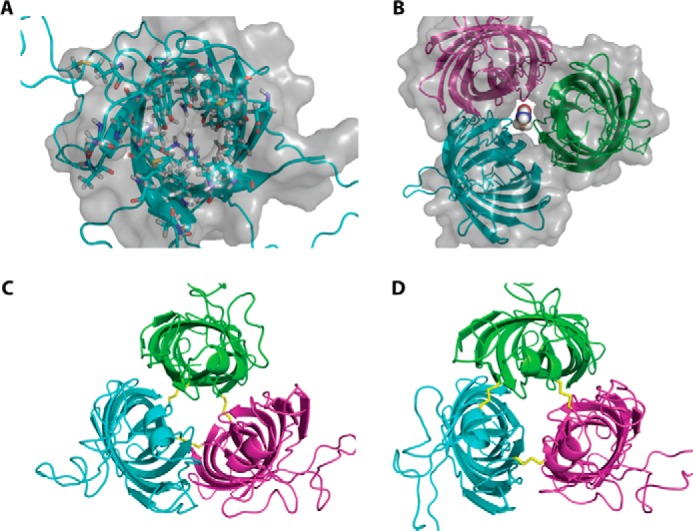
Structure models of monomeric and hypothetical trimeric OprG. A, top view of the NMR structure of OprG (PDB code 2N6L). Residues pointing inside the barrel are shown as ball and stick models and the solvent accessible surface of the barrel as a gray translucent volume. This clearly indicates that there is no luminal channel large enough to transport small amino acids. B, homology model of a hypothetical OprG trimer based on the crystal structure of OmpW (PDB code 2F1V). The solvent accessible surface shows a channel along the trimeric interface large enough to accommodate the amino acid glycine. C, disulfide-linked trimer of T65C–L90C OprG based on the homology model of B. D, disulfide-linked trimer of S128C–S136C OprG based on the homology model of B.
