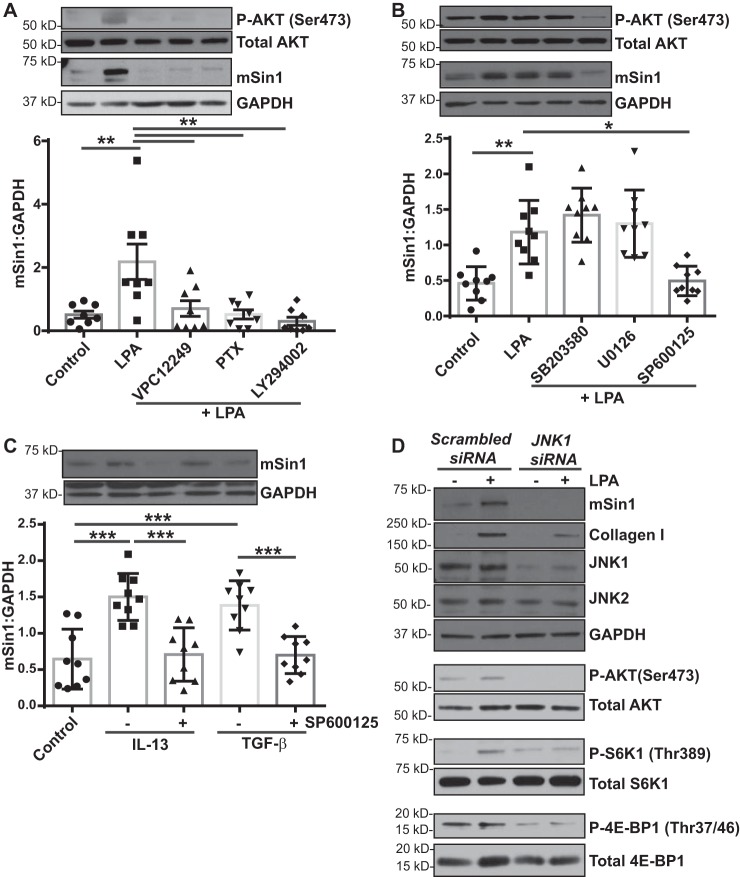
Figure 3.
LPA-mediated induction of the mTORC2 binding partner mSin1 depends on JNK. A, nonfibrotic MCs (n = 8) were pretreated with inhibitors specific to LPA1 (VPC12249), Gi (PTX), or PI3K (LY294002) and then stimulated with LPA (10 μm for 6 h). Cell lysates were immunoblotted with antibodies against phosphorylated and total forms of AKT, mSin1, and GAPDH (loading control). Densitometry analysis of mSin1 was expressed as means ± S.D. **, p < 0.01; n = 9. B, nonfibrotic MCs (n = 9) were pretreated with inhibitors specific to p38MAPK (SB203580), ERK (U0126), and JNK (SP600125) and then stimulated with LPA (10 μm for 6 h). Cell lysates were immunoblotted with antibodies against the phosphorylated and total forms of AKT, mSin1, and GAPDH (loading control). Densitometry analysis of mSin1 was expressed as means ± S.D. **, p < 0.01; *, p < 0.05. C, nonfibrotic MCs (n = 9) were pretreated with a JNK inhibitor (SP600125), followed by stimulation with IL-13 (10 ng/ml) or TGF-β (2 ng/ml) for 16 h. Cell lysates were analyzed for mSin1 by immunoblotting. Densitometry analysis of mSin1 was expressed as means ± S.D. ***, p < 0.001. Statistics: one-way ANOVA; post hoc: Sidak. D, nonfibrotic MCs were transfected with scrambled or JNK1-specific siRNA and then stimulated with LPA (10 μm for 6 h). Cell lysates were immunoblotted with antibodies against mSin1, collagen I, JNK1/2, or phosphorylated and total forms of AKT (Ser-473), p70S6K1 (Thr-389), and 4E-BP1 (Thr-37/46) using GAPDH as a loading control. Representative blots are shown.