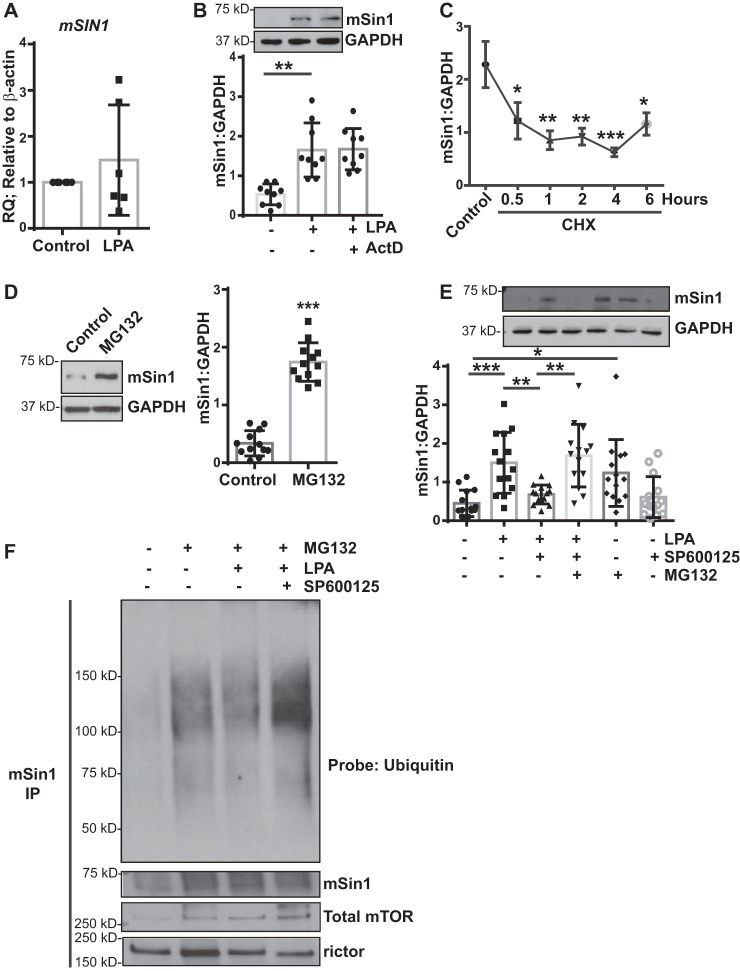
Figure 5.
JNK mediates posttranslational regulation of mSin1 induction. A, nonfibrotic MCs (n = 6) were stimulated with LPA (10 μm for 6 h). RNA lysates were analyzed for mSIN1 by qPCR, using β-actin as the endogenous control. B, nonfibrotic MCs (n = 9) were pretreated with actinomycin D (ActD, 5 μg/ml for 30 min), followed by stimulation with LPA. Cell lysates were immunoblotted for antibodies against mSin1 and GAPDH (loading control). **, p < 0.01. Statistics: one-way ANOVA; post hoc: Sidak. C, nonfibrotic MCs (n = 5) were pretreated with CHX (10 μm) for the indicated periods of time. Protein lysates were subjected to a chase assay and analyzed for mSin1. *, p < 0.05; **, p < 0.01; ***, p < 0.001. D, nonfibrotic MCs (n = 12) were treated with the proteasomal inhibitor MG132 (10 μm for 16 h). Protein lysates were immunoblotted with antibodies against mSin1 and GAPDH (loading control). Densitometric values are represented as means ± S.D. ***, p < 0.001. E, nonfibrotic MCs (n = 14) were pretreated with a proteasomal inhibitor (MG132) or JNK inhibitor (SP600125) or both, followed by stimulation with LPA. Cell lysates were analyzed for mSin1 and GAPDH (loading control). Densitometric values are represented as means ± S.D. *, p < 0.05; **, p < 0.01; ***, p < 0.001. Statistics: one-way ANOVA; post hoc: Sidak. F, nonfibrotic MCs (n = 3) were treated with the proteasomal inhibitor MG132 in the presence or absence of JNK inhibitor II (SP600125) with or without LPA stimulation for 16 h. Cell lysates were immunoprecipitated using antibodies against mSin1. mSin1 immunoprecipitates were immunoblotted with antibodies against pan-ubiquitin, mSin1, rictor, and total mTOR. Representative blots are shown.