Figure 3.
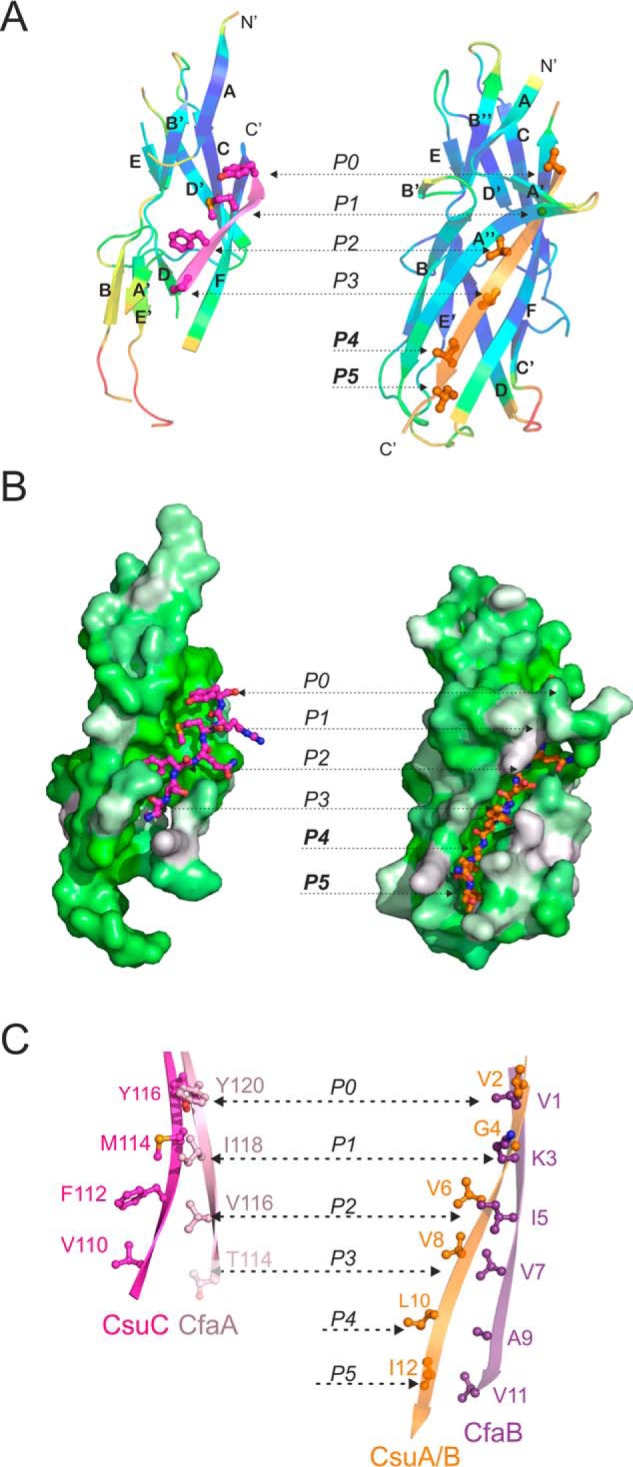
Comparison of the structure and donor strand complementation mechanism of the chaperone-bound and fiber inserted pilins from nonclassical systems. A and B, CsuA/B complemented with the G1 donor strand of CsuC (chaperone-bond conformation) is shown on the left and CsuA/Bsc (fiber-inserted conformation) is shown on the right. A, cartoon representation. CsuA/B is colored by B-factors. G1 and Gd donor strands are colored magenta and orange, respectively; donor residues are shown as ball-and-sticks. B, surface representation. CsuA/B is colored using the Eisenberg hydrophobicity scale. G1 and Gd donor strands are shown as ball-and-stick models. C, comparison of donor motifs in G1 strands of archaic CsuC and alternative CfaA chaperones (left diagram) and Gd strands of the respective pilins, CsuA/B and CfaB (right diagram). The images highlight fragments of structural superpositions of CsuC-CsuA/B and CfaA-CfaB (4Y2O) complexes, and self-complemented CsuA/B and CfaB (3F83) pilins. Donor residues are shown as ball-and-sticks and labeled. Pockets P0–P5 in the acceptor cleft of the pilin are indicated in A–C. Thr-114 in CfaA is shown in pale pink, because it only partially occupies acceptor pocket P3.
