Abstract
Grapes are widely used in the wine and juice industries, which can lead to massive amounts of waste, mostly grape peels and seeds. The antioxidant capacities, total phenolic and flavonoid contents and phenolic profiles of peels and seeds from 30 grape varieties were systemically assessed. The antioxidant activities of fat-soluble, water-soluble and insoluble-bound fractions of grape peels and seeds were evaluated using ferric-reducing antioxidant power and Trolox equivalent antioxidant capacity assays, and their total phenolic contents and total flavonoid contents were determined by the Folin-Ciocalteu method and AlCl3 colorimetry, respectively. It was found that the antioxidant capacities were diverse among different grape peels and seeds. Moreover, several phenolic compounds were identified and quantified, including gallic acid, cyanidin-3-glucoside, epicatechin, catechin gallate, ferulaic acid, rutin and resveratrol, which could contribute to the antioxidant capacities of these grape peels and seeds. Several grape wastes with strong antioxidant activity could be abundant sources of natural bioactive compounds, and have the potential for development into functional foods, food additives and pharmaceuticals.
Keywords: grape, peel, seed, waste, antioxidant capacity, bioactive compounds, phenol, flavonoid
1. Introduction
Grape is a famous fruit all over the world, and is widely used in wine and juice industries, which can lead to massive amounts of wastes, including grape peels and seeds [1]. It is reported that these wastes contain a variety of phytochemicals, especially phenols and flavonoids like anthocyanins, resveratrol, tannin and quercetin [2,3,4,5,6,7,8]. These bioactive components possess various outstanding bioactivities, such as antibacterial, anticancer, antioxidant, anti-inflammation and hepatic and cardiovascular protection effects [9,10,11,12,13], and have great safety and effectiveness advantages in preventing chronic diseases [14,15,16,17]. They can be used as raw materials to produce functional foods, food additives and pharmaceuticals [18,19,20,21,22,23]. Many factors can influence the composition and contents of bioactive compounds in fruits, like genotype, growth environment (soil, water, sunlight, etc.) and maturity, among which genotype usually has the greatest impact [24,25,26]. Thus, we could hypothesize that grapes with diverse genotypes should have different composition and contents of bioactive compounds, so it is worthwhile to assess the antioxidant capacities while determining the phenolic and flavonoid contents of peels and seeds from different grape varieties. In the present study, the antioxidant capacities of peels and seeds from 30 grape varieties were measured, and their total phenolic contents and total flavonoid contents were evaluated. In addition, the phenolic and flavonoid constituents were identified and quantified using HPLC analysis. This should prove helpful for the full utilization of grape peels and seeds.
2. Results and Discussion
2.1. Ferric Reducing Antioxidant Power (FRAP) of the Grape Peels and Seeds
The FRAP was used as one of the indices to assess antioxidant capacities of these grape peels and seeds. The FRAP assay is established on the basis of the ability that antioxidants reduce ferric ions to ferrous ions [27], which is a simple and commonly employed method to evaluate antioxidant capacity [28,29,30]. The FRAP values of these grape peels and seeds are presented in Table 1.
Table 1.
FRAP values of peels and seeds from 30 grape varieties.
| Name of Grapes | Place of Production | Part of Grapes | FARAP Values (μmol Fe(II)/g FW) | |||
|---|---|---|---|---|---|---|
| Fat-Soluble Fraction | Water-Soluble Fraction | Insoluble-Bound Fraction | Total | |||
| Black Grape | Yunnan, China | Peel | 99.407 ± 4.048 | 54.026 ± 1.833 | 0.273 ± 0.024 | 153.706 ± 5.904 |
| Blackcurrant Grape | California, CA, USA | Peel | 161.671 ± 5.628 | 91.100 ± 3.554 | 0.211 ± 0.003 | 252.983 ± 9.185 |
| Flame Grape | Xinjiang, China | Peel | 24.241 ± 2.288 | 40.931 ± 1.694 | 0.286 ± 0.022 | 65.457 ± 4.004 |
| ragrant Green Grape | Yunnan, China | Peel | 6.734 ± 0.364 | 11.407 ± 0.311 | 0.163 ± 0.005 | 18.304 ± 0.680 |
| Golden Finger Grape | California, CA, USA | Peel | 106.886 ± 5.354 | 115.195 ± 0.595 | 0.074 ± 0.005 | 222.155 ± 5.954 |
| Green Grape | Victoria, Australia | Peel | 20.336 ± 0.398 | 22.645 ± 1.450 | 0.245 ± 0.010 | 43.226 ± 1.858 |
| Ito Kyoho Grape | Yunnan, China | Peel | 63.526 ± 4.318 | 40.336 ± 1.193 | 0.170 ± 0.015 | 104.032 ± 5.526 |
| Kyoho Grape | Guangxi, China | Peel | 94.002 ± 2.110 | 26.026 ± 2.239 | 0.614 ± 0.032 | 120.642 ± 4.380 |
| Kyoho Grape | Liaoning, China | Peel | 59.621 ± 1.689 | 15.907 ± 0.655 | 0.164 ± 0.007 | 75.693 ± 2.351 |
| Kyoho Grape | Xinjiang, China | Peel | 90.217 ± 5.724 | 12.407 ± 1.111 | 0.092 ± 0.005 | 102.716 ± 6.840 |
| Kyoho Grape | Yunnan, China | Peel | 75.812 ± 5.004 | 24.264 ± 2.020 | 0.254 ± 0.023 | 100.331 ± 7.047 |
| Pearl Black Grape | Xinjiang, China | Peel | 84.479 ± 2.465 | 53.600 ± 3.536 | 0.144 ± 0.004 | 138.223 ± 6.005 |
| Pearl Green Grape | Xinjiang, China | Peel | 7.288 ± 0.648 | 20.907 ± 0.842 | 0.154 ± 0.013 | 28.350 ± 1.503 |
| Pearl Green Grape | Victoria, Australia | Peel | 36.955 ± 1.041 | 40.883 ± 1.636 | 0.211 ± 0.013 | 78.050 ± 2.689 |
| Red Grape | California, CA, USA | Peel | 42.169 ± 3.351 | 27.741 ± 0.975 | 0.083 ± 0.003 | 69.992 ± 4.329 |
| Red Grape | Guangxi, China | Peel | 35.645 ± 1.221 | 24.764 ± 0.446 | 0.131 ± 0.011 | 60.541 ± 1.678 |
| Red Grape | Xinjiang, China | Peel | 43.812 ± 1.782 | 21.693 ± 1.823 | 0.133 ± 0.013 | 65.638 ± 3.617 |
| Red Grape | Yunnan, China | Peel | 62.336 ± 2.133 | 35.431 ± 2.093 | 0.203 ± 0.005 | 97.969 ± 4.231 |
| Rose Black Grape | Xinjiang, China | Peel | 62.526 ± 3.635 | 65.717 ± 5.437 | 0.387 ± 0.030 | 128.630 ± 9.103 |
| Rose Black Grape | Yunnan, China | Peel | 59.383 ± 2.580 | 39.741 ± 1.034 | 0.408 ± 0.033 | 99.532 ± 3.646 |
| Seedless Black Grape | California, CA, USA | Peel | 106.674 ± 2.619 | 90.669 ± 8.983 | 0.401 ± 0.036 | 197.742 ± 11.638 |
| Seedless Black Grape | Xinjiang, China | Peel | 66.526 ± 2.269 | 67.074 ± 3.610 | 0.219 ± 0.003 | 133.819 ± 5.882 |
| Seedless Dew Grape | Xinjiang, China | Peel | 18.241 ± 1.656 | 31.136 ± 1.262 | 0.295 ± 0.015 | 49.672 ± 2.933 |
| Seedless Green Grape | Xinjiang, China | Peel | 7.264 ± 0.707 | 25.169 ± 1.567 | 0.131 ± 0.010 | 32.564 ± 2.284 |
| Seedless Red Grape | California, CA, USA | Peel | 53.657 ± 0.051 | 71.217 ± 4.113 | 0.094 ± 0.003 | 124.967 ± 4.166 |
| Seedless Red Grape | Victoria, Australia | Peel | 65.669 ± 2.402 | 65.288 ± 5.794 | 0.294 ± 0.003 | 131.251 ± 8.198 |
| Seedless Red Grape | Xinjiang, China | Peel | 24.026 ± 0.664 | 26.002 ± 1.329 | 0.138 ± 0.005 | 50.167 ± 1.998 |
| Seedless Red Grape | Yunnan, China | Peel | 25.669 ± 0.972 | 31.979 ± 1.608 | 0.217 ± 0.005 | 57.864 ± 2.586 |
| Summer Black Grape | Shaanxi, China | Peel | 85.121 ± 6.061 | 72.407 ± 4.772 | 0.233 ± 0.013 | 157.761 ± 10.846 |
| Summer Black Grape | Xinjiang, China | Peel | 56.883 ± 5.017 | 46.312 ± 3.526 | 0.379 ± 0.012 | 103.574 ± 8.555 |
| Black Grape | Yunnan, China | Seed | 692.019 ± 18.217 | 144.767 ± 3.348 | 0.456 ± 0.013 | 837.242 ± 21.578 |
| Ito Kyoho Grape | Yunnan, China | Seed | 301.924 ± 4.439 | 64.148 ± 4.014 | 0.424 ± 0.000 | 366.495 ± 8.452 |
| Kyoho Grape | Xinjiang, China | Seed | 371.448 ± 13.718 | 50.243 ± 3.295 | 0.436 ± 0.023 | 422.127 ± 17.036 |
| Kyoho Grape | Guangxi, China | Seed | 250.876 ± 8.208 | 59.671 ± 3.369 | 1.881 ± 0.184 | 312.429 ± 11.760 |
| Kyoho Grape | Yunnan, China | Seed | 283.638 ± 22.325 | 44.148 ± 3.996 | 0.868 ± 0.052 | 328.654 ± 26.373 |
| Pearl Black Grape | Xinjiang, China | Seed | 726.495 ± 23.487 | 131.243 ± 11.987 | 0.383 ± 0.033 | 858.121 ± 35.507 |
| Red Grape | Yunnan, China | Seed | 502.876 ± 27.668 | 112.862 ± 1.918 | 0.747 ± 0.043 | 616.485 ± 29.629 |
| Red Grape | Guangxi, China | Seed | 351.067 ± 19.144 | 122.576 ± 3.677 | 0.528 ± 0.005 | 474.170 ± 22.825 |
| Red Grape | Xinjiang, China | Seed | 450.305 ± 15.144 | 111.243 ± 4.267 | 0.470 ± 0.027 | 562.018 ± 19.437 |
| Red Grape | California, CA, USA | Seed | 401.924 ± 16.337 | 117.148 ± 3.570 | 1.319 ± 0.068 | 520.390 ± 19.974 |
For the 30 grape peels, the total FRAP values varied from 18.304 ± 0.680 to 252.983 ± 9.185 μmol Fe(II)/g fresh weight (FW) with a 14-fold difference. Blackcurrant Grape (California, CA, USA), Golden Finger Grape (California, CA, USA), Seedless Black Grape (California, CA, USA), Summer Black Grape (Shaanxi, China) and Black Grape (Yunnan, China) possessed the top-five antioxidant capacities, which were 252.983 ± 9.185, 222.155 ± 5.954, 197.742 ± 11.638, 157.761 ± 10.846 and 153.706 ± 5.904 μmol Fe(II)/g FW, respectively. Fragrant Green Grape (Yunnan, China) possessed the lowest antioxidant capacity, which was 18.304 ± 0.680 μmol Fe(II)/g FW. In addition, the ranges of FRAP values for three fractions were in a decreasing order: fat-soluble (6.734 ± 0.364 to 161.671 ± 5.628 μmol Fe(II)/g FW) > water-soluble (11.407 ± 0.311 to 115.195 ± 0.595 μmol Fe(II)/g FW) > insoluble-bound (0.074 ± 0.005 to 0.614 ± 0.032 μmol Fe(II)/g FW) (p = 0.030, p < 0.001, respectively).
For the 10 grape seeds, the total FRAP values varied from 312.429 ± 11.760 to 858.121 ± 35.507 μmol Fe(II)/g FW with a 3-fold difference. Pearl Black Grape (Xinjiang, China), Black Grape (Yunnan, China), Red Grape (Yunnan, China), Red Grape (Xinjiang, China) and Red Grape (California, CA, USA) possessed the top-five antioxidant capacities, which were 858.121 ± 35.507, 837.242 ± 21.578, 616.485 ± 29.629, 562.018 ± 19.437 and 520.390 ± 19.974, respectively. Kyoho Grape (Guangxi, China) possessed the lowest antioxidant capacity, which was 312.429 ± 11.760 μmol Fe(II)/g FW. In addition, the ranges of FRAP values for three fractions were in a decreasing order: fat-soluble (250.876 ± 8.208 to 726.495 ± 23.487 μmol Fe(II)/g FW) > water-soluble (44.148 ± 3.996 to 144.767 ± 3.348 μmol Fe(II)/g FW) > insoluble-bound (0.383 ± 0.033 to 1.881 ± 0.184 μmol Fe(II)/g FW) (p < 0.001, p = 0.038, respectively).
According to the results described above, the FRAP values of fat-soluble fractions were generally higher than those of water-soluble fractions, which were distinctly higher than those of insoluble-bound fractions. These results indicated that the antioxidants responsible for the reducing power of grape peels and seeds were most fat-soluble compounds with some water-soluble and a little insoluble-bound ones. When evaluating total antioxidant capacities of grape peels and seeds, all of the three fractions should be taken into consideration. In addition, the FRAP values of grape seeds were apparently higher than those of grape peels (p < 0.001). As compared to other materials, the FRAP values of the tested grape peels were higher than those of most edible macro-fungi, vegetables, fruits and fruit wastes (peels and seeds) [31,32,33,34], and also higher than those of some wild fruits and edible and wild flowers [35,36]. Moreover, the FRAP values of the tested grape seeds were higher than those of most edible macro-fungi, vegetables, wild fruits, edible and wild flowers, fruits and fruit wastes (peels and seeds) [31,32,33,34,35,36], and higher than those of some medicinal plants [37]. So grape peels and seeds could be abundant resources of natural antioxidants with great potential to produce functional foods, food additives and pharmaceuticals.
2.2. Trolox Equivalent Antioxidant Capacity (TEAC) of the Grape Peels and Seeds
Most natural antioxidants are multifunctional, and the antioxidant capacities of plant samples are generally impacted by multiple factors, such as the extraction solvent, extraction method and measurement method, leading to difficulty to completely demonstrate antioxidant capacities using a single method. Therefore, an authentic antioxidant assessing system requires evaluations of multiple aspects, and it is essential to conduct different experiments to assess the antioxidant activity which might be associated with diverse mechanisms of action [38]. The TEAC assay is a simple, fast, repeatable and widely used method for the evaluation of antioxidant capacity [39,40]. The TEAC assay is on the basis of the capability of antioxidants to scavenge the ABTS•+ radical, and can be used for measuring antioxidant capacities of fat-soluble, water-soluble and insoluble-bound components in the same sample [41]. As reported, vitamin C, vitamin E, butylated hydroxytoluene, butylated hydroxyanisole and Trolox were often applied as reference standards [42,43]. Here, Trolox was employed. The TEAC values of the peels and seeds from 30 grape varieties are displayed in Table 2.
Table 2.
TEAC values of peels and seeds from 30 grape varieties.
| Name of Grapes | Place of Production | Part of Grapes | TEAC Values (μmol Trolox/g FW) | |||
|---|---|---|---|---|---|---|
| Fat-Soluble Fraction | Water-Soluble Fraction | Insoluble-Bound Fraction | Total | |||
| Black Grape | Yunnan, China | Peel | 58.264 ± 2.194 | 23.4742 ± 0.637 | 0.315 ± 0.030 | 82.053 ± 2.861 |
| Blackcurrant Grape | California, CA, USA | Peel | 84.463 ± 1.361 | 39.1097 ± 1.59 | 0.167 ± 0.015 | 123.740 ± 2.969 |
| Flame Grape | Xinjiang, China | Peel | 14.572 ± 1.001 | 9.0073 ± 0.140 | 0.209 ± 0.016 | 23.788 ± 1.157 |
| Fragrant Green Grape | Yunnan, China | Peel | 2.293 ± 0.133 | 2.7259 ± 0.061 | 0.156 ± 0.015 | 5.1760 ± 0.209 |
| Golden Finger Grape | California, CA, USA | Peel | 68.596 ± 5.519 | 29.4711 ± 0.381 | 0.039 ± 0.003 | 98.106 ± 5.902 |
| Green Grape | Victoria, Australia | Peel | 13.165 ± 0.524 | 7.4739 ± 0.314 | 0.166 ± 0.016 | 20.804 ± 0.854 |
| Ito Kyoho Grape | Yunnan, China | Peel | 34.777 ± 2.078 | 16.1091 ± 0.714 | 0.242 ± 0.023 | 51.128 ± 2.815 |
| Kyoho Grape | Guangxi, China | Peel | 31.151 ± 1.088 | 3.8408 ± 0.206 | 0.138 ± 0.003 | 35.130 ± 1.297 |
| Kyoho Grape | Liaoning, China | Peel | 42.555 ± 0.447 | 5.3211 ± 0.081 | 0.069 ± 0.003 | 47.945 ± 0.531 |
| Kyoho Grape | Xinjiang, China | Peel | 50.852 ± 3.082 | 12.8820 ± 0.979 | 0.383 ± 0.029 | 64.117 ± 4.090 |
| Kyoho Grape | Yunnan, China | Peel | 38.092 ± 1.912 | 12.0422 ± 1.146 | 0.163 ± 0.008 | 50.297 ± 3.065 |
| Pearl Black Grape | Xinjiang, China | Peel | 48.202 ± 0.567 | 30.2866 ± 2.763 | 0.075 ± 0.005 | 78.563 ± 3.336 |
| Pearl Green Grape | Xinjiang, China | Peel | 3.267 ± 0.254 | 6.0273 ± 0.026 | 0.141 ± 0.013 | 9.435 ± 0.293 |
| Pearl Green Grape | Victoria, Australia | Peel | 21.066 ± 0.750 | 20.9262 ± 0.525 | 0.105 ± 0.010 | 42.097 ± 1.285 |
| Red Grape | California, CA, USA | Peel | 34.654 ± 0.605 | 15.7459 ± 0.266 | 0.158 ± 0.010 | 50.557 ± 0.881 |
| Red Grape | Guangxi, China | Peel | 21.460 ± 0.485 | 6.5821 ± 0.043 | 0.098 ± 0.008 | 28.141 ± 0.536 |
| Red Grape | Xinjiang, China | Peel | 26.268 ± 2.316 | 9.8911 ± 0.620 | 0.134 ± 0.011 | 36.293 ± 2.947 |
| Red Grape | Yunnan, China | Peel | 24.813 ± 1.079 | 15.1921 ± 0.525 | 0.028 ± 0.002 | 40.033 ± 1.606 |
| Rose Black Grape | Xinjiang, China | Peel | 35.038 ± 1.647 | 29.0777 ± 2.282 | 0.237 ± 0.016 | 64.353 ± 3.944 |
| Rose Black Grape | Yunnan, China | Peel | 30.936 ± 1.431 | 19.7177 ± 0.469 | 0.216 ± 0.009 | 50.869 ± 1.909 |
| Seedless Black Grape | California, CA, USA | Peel | 34.388 ± 0.258 | 27.5440 ± 1.616 | 0.116 ± 0.008 | 62.047 ± 1.882 |
| Seedless Black Grape | Xinjiang, China | Peel | 62.835 ± 0.877 | 38.0697 ± 2.950 | 0.246 ± 0.012 | 101.151 ± 3.839 |
| Seedless Dew Grape | Xinjiang, China | Peel | 11.461 ± 0.428 | 7.9079 ± 0.043 | 0.252 ± 0.003 | 19.621 ± 0.473 |
| Seedless Green Grape | Xinjiang, China | Peel | 4.579 ± 0.363 | 10.3781 ± 0.402 | 0.083 ± 0.008 | 15.040 ± 0.773 |
| Seedless Red Grape | California, CA, USA | Peel | 16.143 ± 0.473 | 13.5667 ± 0.159 | 0.150 ± 0.010 | 29.860 ± 0.642 |
| Seedless Red Grape | Victoria, Australia | Peel | 14.900 ± 0.758 | 11.7903 ± 0.731 | 0.033 ± 0.003 | 26.724 ± 1.491 |
| Seedless Red Grape | Xinjiang, China | Peel | 29.336 ± 1.267 | 28.2014 ± 1.974 | 0.044 ± 0.003 | 57.582 ± 3.243 |
| Seedless Red Grape | Yunnan, China | Peel | 36.584 ± 1.474 | 21.6865 ± 1.676 | 0.103 ± 0.005 | 58.374 ± 3.155 |
| Summer Black Grape | Shaanxi, China | Peel | 51.789 ± 1.878 | 30.2866 ± 2.195 | 0.167 ± 0.013 | 82.242 ± 4.086 |
| Summer Black Grape | Xinjiang, China | Peel | 31.496 ± 2.283 | 28.6149 ± 1.900 | 0.246 ± 0.009 | 60.358 ± 4.192 |
| Black Grape | Yunnan, China | Seed | 329.773 ± 5.710 | 62.5184 ± 0.510 | 0.287 ± 0.015 | 392.577 ± 6.236 |
| Ito Kyoho Grape | Yunnan, China | Seed | 181.739 ± 1.029 | 31.7674 ± 1.296 | 0.295 ± 0.023 | 213.802 ± 2.348 |
| Kyoho Grape | Xinjiang, China | Seed | 231.921 ± 9.528 | 24.5545 ± 1.907 | 0.207 ± 0.005 | 256.682 ± 11.440 |
| Kyoho Grape | Guangxi, China | Seed | 192.411 ± 9.735 | 38.4204 ± 1.567 | 1.320 ± 0.071 | 232.152 ± 11.373 |
| Kyoho Grape | Yunnan, China | Seed | 176.024 ± 8.804 | 31.2584 ± 1.739 | 0.532 ± 0.031 | 207.815 ± 10.573 |
| Pearl Black Grape | Xinjiang, China | Seed | 409.190 ± 19.195 | 64.1050 ± 0.106 | 0.159 ± 0.003 | 473.454 ± 19.303 |
| Red Grape | Yunnan, China | Seed | 274.455 ± 11.707 | 55.1126 ± 1.364 | 0.587 ± 0.015 | 330.155 ± 13.086 |
| Red Grape | Guangxi, China | Seed | 199.613 ± 6.407 | 54.3559 ± 1.308 | 0.380 ± 0.005 | 254.349 ± 7.720 |
| Red Grape | Xinjiang, China | Seed | 246.250 ± 7.908 | 47.3937 ± 0.889 | 0.266 ± 0.007 | 293.910 ± 8.804 |
| Red Grape | California, CA, USA | Seed | 229.045 ± 7.867 | 62.7832 ± 0.696 | 0.520 ± 0.048 | 292.349 ± 8.610 |
For the 30 grape peels, the total TEAC values ranged from 5.176 ± 0.209 to 123.740 ± 2.969 μmol Trolox/g FW with a 24-fold difference. Blackcurrant Grape (California, CA, USA), Seedless Black Grape (Xinjiang, China), Golden Finger Grape (California, CA, USA), Summer Black Grape (Shaanxi, China) and Black Grape (Yunnan, China) possessed the top-five free radical scavenging capacities, which were 123.740 ± 2.969, 101.151 ± 3.839, 98.106 ± 5.902, 82.242 ± 4.086 and 82.053 ± 2.861 μmol Trolox/g FW, respectively. Fragrant Green Grape (Yunnan, China) possessed the lowest free radical scavenging capacity, which was 5.176 ± 0.209 μmol Trolox/g FW. In addition, the ranges of TEAC values for three fractions were in a decreasing order: fat-soluble (2.293 ± 0.133 to 84.463 ± 1.361 μmol trolox/g FW) > water-soluble (2.726 ± 0.061 to 39.110 ± 1.592 μmol Trolox/g FW) > insoluble-bound (0.028 ± 0.002 to 0.383 ± 0.029 μmol trolox/g FW) (p < 0.001, p < 0.001, respectively).
For the 10 grape seeds, the total TEAC values ranged from 207.815 ± 10.573 to 473.454 ± 19.303 μmol Trolox/g FW with a 2-fold difference. Pearl Black Grape (Xinjiang, China), Black Grape (Yunnan, China), Red Grape (Yunnan, China), Red Grape (Xinjiang, China) and Red Grape (California, CA, USA) possessed the top-five free radical scavenging capacities, which were 473.454 ± 19.303, 392.577 ± 6.236, 330.155 ± 13.086, 293.910 ± 8.804 and 292.349 ± 8.610 μmol Trolox/g FW, respectively. Kyoho Grape (Yunnan, China) possessed the lowest free radical scavenging capacity, which was 207.815 ± 10.573 μmol Trolox/g FW. In addition, the ranges of TEAC values for three fractions were in a decreasing order: fat-soluble (181.739 ± 1.029 to 409.190 ± 19.195 μmol trolox/g FW) > water-soluble (24.555 ± 1.907 to 64.105 ± 0.106 μmol trolox/g FW) > insoluble-bound (0.159 ± 0.003 to 1.320 ± 0.071 μmol trolox/g FW) (p < 0.001, p = 0.023, respectively).
As seen from the description before, the TEAC values of fat-soluble fractions were generally higher than those of water-soluble fractions, which were distinctly higher than those of insoluble-bound fractions. It meant that the antioxidants, which were responsible for the free radical scavenging activities of grape peels and seeds, were most fat-soluble compounds with some water soluble and a little insoluble-bound ones. When the total antioxidant capacities of grape peels and seeds are about to be assessed, three fractions should all be counted in. In addition, the TEAC values of the grape seeds were extremely higher than those of the grape peels (p < 0.001). Besides, the TEAC values of the grape peels were higher than those of most edible macro-fungi, vegetables, fruits and fruit waste (peels and seeds) [31,32,33,34], and higher than those of some wild fruits and edible and wild flowers [35,36]. Furthermore, the TEAC values of the grape seeds were higher than those of most edible macro-fungi, vegetables, edible and wild flowers, fruits and fruit waste (peels and seeds) [31,32,33,34,35,36], and higher than those of some wild fruits and medicinal plants [37], so grape peels and seeds could be developed into functional foods, food additives and pharmaceuticals regarding antioxidants.
2.3. Total Phenolic Contents (TPC) of 30 Grape Peels and 10 Grape Seeds
The TPC values of these grape peels and seeds were determined by the Folin-Ciocalteu method, which is based on the reaction that electrons are transferred from phenolic compounds to the Folin-Ciocalteu reagent in alkaline medium, and is a simple, rapid and reproducible method [44]. The TPC values of these grape peels and seeds are given in Table 3.
Table 3.
TPC values of peels and seeds from 30 grape varieties.
| Name of Grapes | Place of Production | Part of Grapes | TPC Values (mg GAE/g FW) | |||
|---|---|---|---|---|---|---|
| Fat-Soluble Fraction | Water-Soluble Fraction | Insoluble-Bound Fraction | Total | |||
| Black Grape | Yunnan, China | Peel | 8.992 ± 0.646 | 5.516 ± 0.091 | 0.066 ± 0.006 | 14.574 ± 0.742 |
| Blackcurrant Grape | California, CA, USA | Peel | 16.529 ± 0.463 | 9.171 ± 0.430 | 0.025 ± 0.001 | 25.724 ± 0.894 |
| Flame Grape | Xinjiang, China | Peel | 2.5485 ± 0.173 | 3.782 ± 0.144 | 0.029 ± 0.002 | 6.356 ± 0.319 |
| Fragrant Green Grape | Yunnan, China | Peel | 0.811 ± 0.025 | 0.754 ± 0.037 | 0.024 ± 0.001 | 1.588 ± 0.062 |
| Golden Finger Grape | California, CA, USA | Peel | 6.443 ± 0.488 | 6.673 ± 0.024 | 0.015 ± 0.001 | 13.131 ± 0.514 |
| Green Grape | Victoria, Australia | Peel | 2.530 ± 0.028 | 2.366 ± 0.087 | 0.029 ± 0.002 | 4.925 ± 0.118 |
| Ito Kyoho Grape | Yunnan, China | Peel | 6.778 ± 0.300 | 3.774 ± 0.139 | 0.047 ± 0.004 | 10.599 ± 0.444 |
| Kyoho Grape | Guangxi, China | Peel | 6.809 ± 0.176 | 1.177 ± 0.061 | 0.018 ± 0.001 | 8.003 ± 0.239 |
| Kyoho Grape | Liaoning, China | Peel | 8.719 ± 0.341 | 6.752 ± 0.664 | 0.012 ± 0.001 | 15.483 ± 1.006 |
| Kyoho Grape | Xinjiang, China | Peel | 9.341 ± 0.517 | 2.935 ± 0.095 | 0.072 ± 0.002 | 12.348 ± 0.614 |
| Kyoho Grape | Yunnan, China | Peel | 7.689 ± 0.713 | 2.962 ± 0.151 | 0.036 ± 0.003 | 10.687 ± 0.866 |
| Pearl Black Grape | Xinjiang, China | Peel | 8.374 ± 0.210 | 6.944 ± 0.686 | 0.020 ± 0.001 | 15.338 ± 0.897 |
| Pearl Green Grape | Xinjiang, China | Peel | 1.138 ± 0.014 | 1.872 ± 0.083 | 0.027 ± 0.002 | 3.037 ± 0.099 |
| Pearl Green Grape | Victoria, Australia | Peel | 4.189 ± 0.089 | 4.618 ± 0.148 | 0.027 ± 0.002 | 8.833 ± 0.239 |
| Red Grape | California, CA, USA | Peel | 6.624 ± 0.233 | 3.528 ± 0.178 | 0.037 ± 0.001 | 10.189 ± 0.412 |
| Red Grape | Guangxi, China | Peel | 4.006 ± 0.400 | 2.331 ± 0.175 | 0.022 ± 0.001 | 6.359 ± 0.575 |
| Red Grape | Xinjiang, China | Peel | 4.935 ± 0.242 | 2.398 ± 0.043 | 0.029 ± 0.001 | 7.362 ± 0.286 |
| Red Grape | Yunnan, China | Peel | 4.683 ± 0.325 | 3.532 ± 0.084 | 0.011 ± 0.001 | 8.226 ± 0.410 |
| Rose Black Grape | Xinjiang, China | Peel | 6.524 ± 0.295 | 6.171 ± 0.486 | 0.043 ± 0.004 | 12.738 ± 0.786 |
| Rose Black Grape | Yunnan, China | Peel | 6.319 ± 0.560 | 4.669 ± 0.086 | 0.045 ± 0.002 | 11.032 ± 0.648 |
| Seedless Black Grape | California, CA, USA | Peel | 6.758 ± 0.193 | 6.512 ± 0.345 | 0.023 ± 0.000 | 13.293 ± 0.538 |
| Seedless Black Grape | Xinjiang, China | Peel | 11.426 ± 0.278 | 8.679 ± 0.703 | 0.048 ± 0.002 | 20.153 ± 0.983 |
| Seedless Dew Grape | Xinjiang, China | Peel | 2.372 ± 0.146 | 2.626 ± 0.185 | 0.041 ± 0.001 | 5.039 ± 0.332 |
| Seedless Green Grape | Xinjiang, China | Peel | 2.795 ± 0.026 | 2.774 ± 0.099 | 0.025 ± 0.002 | 5.594 ± 0.126 |
| Seedless Red Grape | California, CA, USA | Peel | 2.992 ± 0.052 | 2.933 ± 0.041 | 0.028 ± 0.002 | 5.953 ± 0.095 |
| Seedless Red Grape | Victoria, Australia | Peel | 2.839 ± 0.144 | 2.764 ± 0.113 | 0.016 ± 0.000 | 5.619 ± 0.257 |
| Seedless Red Grape | Xinjiang, China | Peel | 5.667 ± 0.086 | 6.553 ± 0.308 | 0.012 ± 0.001 | 12.232 ± 0.395 |
| Seedless Red Grape | Yunnan, China | Peel | 6.599 ± 0.110 | 6.547 ± 0.611 | 0.023 ± 0.002 | 13.169 ± 0.723 |
| Summer Black Grape | Shaanxi, China | Peel | 8.624 ± 0.535 | 6.169 ± 0.342 | 0.029 ± 0.002 | 14.822 ± 0.879 |
| Summer Black Grape | Xinjiang, China | Peel | 5.606 ± 0.078 | 5.036 ± 0.318 | 0.043 ± 0.001 | 10.685 ± 0.397 |
| Black Grape | Yunnan, China | Seed | 57.169 ± 0.954 | 13.150 ± 0.249 | 0.057 ± 0.004 | 70.376 ± 1.207 |
| Ito Kyoho Grape | Yunnan, China | Seed | 29.966 ± 0.098 | 7.300 ± 0.403 | 0.045 ± 0.002 | 37.311 ± 0.503 |
| Kyoho Grape | Xinjiang, China | Seed | 37.527 ± 0.483 | 5.593 ± 0.365 | 0.054 ± 0.005 | 43.174 ± 0.853 |
| Kyoho Grape | Guangxi, China | Seed | 29.998 ± 1.621 | 7.073 ± 0.139 | 0.214 ± 0.009 | 37.285 ± 1.769 |
| Kyoho Grape | Yunnan, China | Seed | 28.584 ± 2.017 | 5.947 ± 0.411 | 0.098 ± 0.006 | 34.628 ± 2.435 |
| Pearl Black Grape | Xinjiang, China | Seed | 58.372 ± 0.692 | 12.833 ± 0.069 | 0.039 ± 0.001 | 71.244 ± 0.762 |
| Red Grape | Yunnan, China | Seed | 44.714 ± 1.636 | 10.967 ± 0.269 | 0.090 ± 0.008 | 55.771 ± 1.912 |
| Red Grape | Guangxi, China | Seed | 33.917 ± 1.436 | 11.333 ± 0.279 | 0.062 ± 0.006 | 45.312 ± 1.722 |
| Red Grape | Xinjiang, China | Seed | 40.811 ± 1.199 | 10.451 ± 0.374 | 0.053 ± 0.005 | 51.315 ± 1.578 |
| Red Grape | California, CA, USA | Seed | 37.104 ± 1.315 | 11.971 ± 0.253 | 0.095 ± 0.002 | 49.170 ± 1.570 |
For the 30 grape peels, the total TPC values varied from 1.588 ± 0.062 to 25.724 ± 0.894 mg GAE/g FW with a 16-fold difference. Blackcurrant Grape (California, CA, USA), Seedless Black Grape (Xinjiang, China), Kyoho Grape (Liaoning, China), Pearl Black Grape (Xinjiang, China) and Summer Black Grape (Shaanxi, China) had the top-five total phenolic contents, which were 25.724 ± 0.894, 20.153 ± 0.983, 15.483 ± 1.006, 15.338 ± 0.897 and 14.822 ± 0.879 mg GAE/g FW, respectively. Fragrant Green Grape (Yunnan, China) had the lowest total phenolic content, which was 1.588 ± 0.062 mg GAE/g FW. In addition, the ranges of TPC values for three fractions were in a decreasing order: fat-soluble (0.811 ± 0.025 to 16.528 ± 0.463 mg GAE/g FW) > water-soluble (0.754 ± 0.037 to 9.1705 ± 0.4299 mg GAE/g FW) > insoluble-bound (0.011 ± 0.001 to 0.072 ± 0.002 mg GAE/g FW) (p = 0.009, p < 0.001, respectively).
For the 10 grape seeds, the total TPC values varied from 34.628 ± 2.435 to 71.244 ± 0.762 mg GAE/g FW with a 2-fold difference. Pearl Black Grape (Xinjiang, China), Black Grape (Yunnan, China), Red Grape (Yunnan, China), Red Grape (Xinjiang, China) and Red Grape (California, CA, USA) had the top-five total phenolic contents, which were 71.244 ± 0.762, 70.376 ± 1.207, 55.771 ± 1.912, 51.315 ± 1.578 and 49.170 ± 1.570 mg GAE/g FW, respectively. Kyoho Grape (Yunnan, China) had the lowest total phenolic content, which was 34.628 ± 2.435 mg GAE/g FW. In addition, the ranges of TPC values for three fractions were in a decreasing order: fat-soluble (28.584 ± 2.017 to 58.372 ± 0.692 mg GAE/g FW) > water-soluble (5.593 ± 0.365 to 13.150 ± 0.249 mg GAE/g FW) > insoluble-bound (0.039 ± 0.001 to 0.214 ± 0.009 mg GAE/g FW) (p < 0.01, p = 0.02, respectively).
Based on the above demonstration, the TPC values of the grape seeds were drastically higher than those of the grape peels (p < 0.001). Besides, the TPC values of the grape peels were higher than those of most edible macro-fungi, vegetables, fruits and fruit waste (peels and seeds) [31,32,33,34], and higher than those of some wild fruits and edible and wild flowers [35,36]. Moreover, the TPC values of the grape seeds were higher than those of most edible macro-fungi, vegetables, edible and wild flowers, wild fruits, fruits and fruit wastes (peels and seeds) [31,32,33,34,35,36], and higher than those of some medicinal plants [37], so grape peels and seeds could be used to extract phenols with further applications in the functional food, pharmaceutical, food additive and cosmetic industries. Furthermore, it should be pointed out that some non-phenolic components such as organic acids and sugars, which also possess reducing capacity, could affect the measurement of total phenolic contents determined by the Folin–Ciocalteu method, leading to overestimated total phenolic contents [45,46]. In addition, varied phenols might response to the Folin–Ciocalteu reagent differently and several flavonoids present low responses, which might cause an underestimate of total phenolic contents [47,48,49].
2.4. Total Flavonoid Contents (TFC) of the Grape Peels and Seeds
The TFC values of these grape peels and seeds were estimated by the AlCl3 colorimetry method according to the literature reported by Kalia et al., which is based on the reaction that the 3-hydroxy-4-hydroxyl or 5-hydroxy-4-carbonyl or o-2-phenolic hydroxyl of flavonoids is combined with Al3+ to form a red complex under an alkaline condition, and is a simple, rapid and repeatable method [50]. The TFC values of these grape peels and seeds are given in Table 4.
Table 4.
TFC values of peels and seeds from 30 grape varieties.
| Name of Grapes | Place of Production | Part of Grapes | TFC Values (mg QE/g FW) | |||
|---|---|---|---|---|---|---|
| Fat-Soluble Fraction | Water-Soluble Fraction | Insoluble-Bound Fraction | Total | |||
| Black Grape | Yunnan, China | Peel | 0.688 ± 0.021 | 0.260 ± 0.009 | 0.014 ± 0.001 | 0.962 ± 0.031 |
| Blackcurrant Grape | California, CA, USA | Peel | 1.017 ± 0.087 | 0.381 ± 0.003 | 0.010 ± 0.000 | 1.408 ± 0.091 |
| Flame Grape | Xinjiang, China | Peel | 0.175 ± 0.011 | 0.139 ± 0.005 | 0.010 ± 0.000 | 0.324 ± 0.016 |
| Fragrant Green Grape | Yunnan, China | Peel | 0.320 ± 0.027 | 0.042 ± 0.001 | 0.081 ± 0.004 | 0.443 ± 0.032 |
| Golden Finger Grape | California, CA, USA | Peel | 0.760 ± 0.059 | 0.362 ± 0.025 | 0.008 ± 0.000 | 1.130 ± 0.084 |
| Green Grape | Victoria, Australia | Peel | 0.232 ± 0.011 | 0.075 ± 0.002 | 0.010 ± 0.001 | 0.318 ± 0.014 |
| Ito Kyoho Grape | Yunnan, China | Peel | 0.302 ± 0.025 | 0.090 ± 0.005 | 0.055 ± 0.002 | 0.448 ± 0.032 |
| Kyoho Grape | Guangxi, China | Peel | 0.425 ± 0.017 | 0.072 ± 0.002 | 0.013 ± 0.001 | 0.510 ± 0.020 |
| Kyoho Grape | Liaoning, China | Peel | 0.326 ± 0.022 | 0.049 ± 0.003 | 0.008 ± 0.000 | 0.384 ± 0.026 |
| Kyoho Grape | Xinjiang, China | Peel | 0.402 ± 0.023 | 0.071 ± 0.006 | 0.016 ± 0.001 | 0.488 ± 0.029 |
| Kyoho Grape | Yunnan, China | Peel | 0.229 ± 0.023 | 0.063 ± 0.005 | 0.012 ± 0.001 | 0.304 ± 0.029 |
| Pearl Black Grape | Xinjiang, China | Peel | 0.398 ± 0.020 | 0.133 ± 0.011 | 0.059 ± 0.001 | 0.590 ± 0.032 |
| Pearl Green Grape | Xinjiang, China | Peel | 0.109 ± 0.002 | 0.052 ± 0.002 | 0.015 ± 0.001 | 0.176 ± 0.005 |
| Pearl Green Grape | Victoria, Australia | Peel | 0.192 ± 0.002 | 0.074 ± 0.002 | 0.011 ± 0.000 | 0.276 ± 0.004 |
| Red Grape | California, CA, USA | Peel | 0.504 ± 0.023 | 0.115 ± 0.006 | 0.013 ± 0.001 | 0.633 ± 0.029 |
| Red Grape | Guangxi, China | Peel | 0.343 ± 0.019 | 0.104 ± 0.005 | 0.011 ± 0.000 | 0.458 ± 0.024 |
| Red Grape | Xinjiang, China | Peel | 0.283 ± 0.018 | 0.076 ± 0.004 | 0.009 ± 0.000 | 0.368 ± 0.022 |
| Red Grape | Yunnan, China | Peel | 0.238 ± 0.008 | 0.065 ± 0.005 | 0.010 ± 0.001 | 0.313 ± 0.013 |
| Rose Black Grape | Xinjiang, China | Peel | 0.425 ± 0.010 | 0.212 ± 0.017 | 0.012 ± 0.001 | 0.649 ± 0.027 |
| Rose Black Grape | Yunnan, China | Peel | 0.326 ± 0.015 | 0.132 ± 0.006 | 0.010 ± 0.001 | 0.468 ± 0.022 |
| Seedless Black Grape | California, CA, USA | Peel | 0.326 ± 0.028 | 0.197 ± 0.004 | 0.012 ± 0.001 | 0.535 ± 0.033 |
| Seedless Black Grape | Xinjiang, China | Peel | 0.642 ± 0.031 | 0.331 ± 0.026 | 0.009 ± 0.000 | 0.982 ± 0.056 |
| Seedless Dew Grape | Xinjiang, China | Peel | 0.163 ± 0.003 | 0.092 ± 0.003 | 0.012 ± 0.001 | 0.266 ± 0.007 |
| Seedless Green Grape | Xinjiang, China | Peel | 0.126 ± 0.009 | 0.061 ± 0.000 | 0.012 ± 0.001 | 0.198 ± 0.010 |
| Seedless Red Grape | California, CA, USA | Peel | 0.199 ± 0.005 | 0.075 ± 0.002 | 0.017 ± 0.000 | 0.291 ± 0.006 |
| Seedless Red Grape | Victoria, Australia | Peel | 0.226 ± 0.010 | 0.091 ± 0.003 | 0.008 ± 0.000 | 0.325 ± 0.013 |
| Seedless Red Grape | Xinjiang, China | Peel | 0.321 ± 0.013 | 0.106 ± 0.000 | 0.008 ± 0.001 | 0.435 ± 0.014 |
| Seedless Red Grape | Yunnan, China | Peel | 0.317 ± 0.001 | 0.125 ± 0.010 | 0.009 ± 0.000 | 0.451 ± 0.011 |
| Summer Black Grape | Shaanxi, China | Peel | 0.317 ± 0.015 | 0.111 ± 0.008 | 0.014 ± 0.001 | 0.441 ± 0.023 |
| Summer Black Grape | Xinjiang, China | Peel | 0.403 ± 0.010 | 0.196 ± 0.015 | 0.011 ± 0.001 | 0.609 ± 0.025 |
| Black Grape | Yunnan, China | Seed | 1.126 ± 0.044 | 0.173 ± 0.003 | 0.041 ± 0.001 | 1.339 ± 0.048 |
| Ito Kyoho Grape | Yunnan, China | Seed | 2.989 ± 0.017 | 0.109 ± 0.005 | 0.024 ± 0.000 | 3.122 ± 0.022 |
| Kyoho Grape | Xinjiang, China | Seed | 3.786 ± 0.182 | 0.078 ± 0.006 | 0.020 ± 0.001 | 3.884 ± 0.189 |
| Kyoho Grape | Guangxi, China | Seed | 2.636 ± 0.238 | 0.096 ± 0.004 | 0.033 ± 0.002 | 2.765 ± 0.245 |
| Kyoho Grape | Yunnan, China | Seed | 1.165 ± 0.051 | 0.074 ± 0.006 | 0.023 ± 0.002 | 1.262 ± 0.059 |
| Pearl Black Grape | Xinjiang, China | Seed | 3.378 ± 0.167 | 0.101 ± 0.002 | 0.146 ± 0.006 | 3.626 ± 0.176 |
| Red Grape | Yunnan, China | Seed | 3.792 ± 0.211 | 0.126 ± 0.001 | 0.038 ± 0.001 | 3.957 ± 0.213 |
| Red Grape | Guangxi, China | Seed | 1.165 ± 0.022 | 0.157 ± 0.010 | 0.040 ± 0.002 | 1.361 ± 0.033 |
| Red Grape | Xinjiang, China | Seed | 2.536 ± 0.227 | 0.114 ± 0.000 | 0.030 ± 0.001 | 2.680 ± 0.227 |
| Red Grape | California, CA, USA | Seed | 1.024 ± 0.044 | 0.086 ± 0.008 | 0.020 ± 0.002 | 1.130 ± 0.054 |
For the 30 grape peels, the total TFC values ranged from 0.176 ± 0.005 to 1.408 ± 0.091 mg QE/g FW with an 8-fold difference. Blackcurrant Grape (California, CA, USA), Golden Finger Grape (California, CA, USA), Seedless Black Grape (Xinjiang, China), Black Grape (Yunnan, China) and Rose Black Grape (Xinjiang, China) had the top-five total flavonoid contents, which were 1.408 ± 0.091, 1.130 ± 0.084, 0.982 ± 0.056, 0.962 ± 0.031 and 0.649 ± 0.027 mg QE/g FW, respectively. Pearl Green Grape (Xinjiang, China) had the lowest total flavonoid content, which was 0.176 ± 0.005 mg QE/g FW. In addition, the ranges of TPC values for three fractions were in a decreasing order: fat-soluble (0.109 ± 0.002 to 1.017 ± 0.087 mg QE/g FW) > water-soluble (0.042 ± 0.001 to 0.381 ± 0.003 mg QE/g FW) > insoluble-bound (0.008 ± 0.000 mg QE/g FW to 0.081 ± 0.004 mg QE/g FW) (p < 0.001, p = 0.001, respectively).
For the 10 grape seeds, the total TFC values ranged from 1.130 ± 0.054 mg QE/g FW to 3.957 ± 0.213 mg QE/g FW with a 4-fold difference. Red Grape (Yunnan, China), Kyoho Grape (Xinjiang, China), Pearl Black Grape (Xinjiang, China), Ito Kyoho Grape(Yunnan, China) and Kyoho Grape (Guangxi, China) had the top-five total flavonoid contents, which were 3.957 ± 0.213, 3.884 ± 0.189, 3.626 ± 0.176, 3.122 ± 0.022 and 2.765 ± 0.245 mg QE/g FW, respectively. Red Grape (California, CA, USA) had the lowest total flavonoid content, which was 1.130 ± 0.054 mg QE/g FW. In addition, the ranges of TPC values for three fractions were in a decreasing order: fat-soluble (1.024 ± 0.044 to 3.792 ± 0.211 mg QE/g FW) > water-soluble (0.074 ± 0.006 mg QE/g FW to 0.173 ± 0.003 mg QE/g FW) > insoluble-bound (0.020 ± 0.001 to 0.146 ± 0.006 mg QE/g FW) (p < 0.001, p = 0.815, respectively).
As illustrated before, the TFC values of the grape seeds were higher than those of the grape peels (p < 0.001). Both of the TFC values of the grape peels and seeds were lower than those of most medicinal plants and some common plant/tree waste [51,52]. Moreover, extracts with higher TPC values did not always have higher TFC values, different extracts contained different levels of TFC as a portion of phenols [51,52,53]. So it should be pointed out that grape peels and seeds were valuable resources of natural phenols but not flavonoids.
2.5. Correlations between Total FRAP, TEAC, TPC and TFC Values
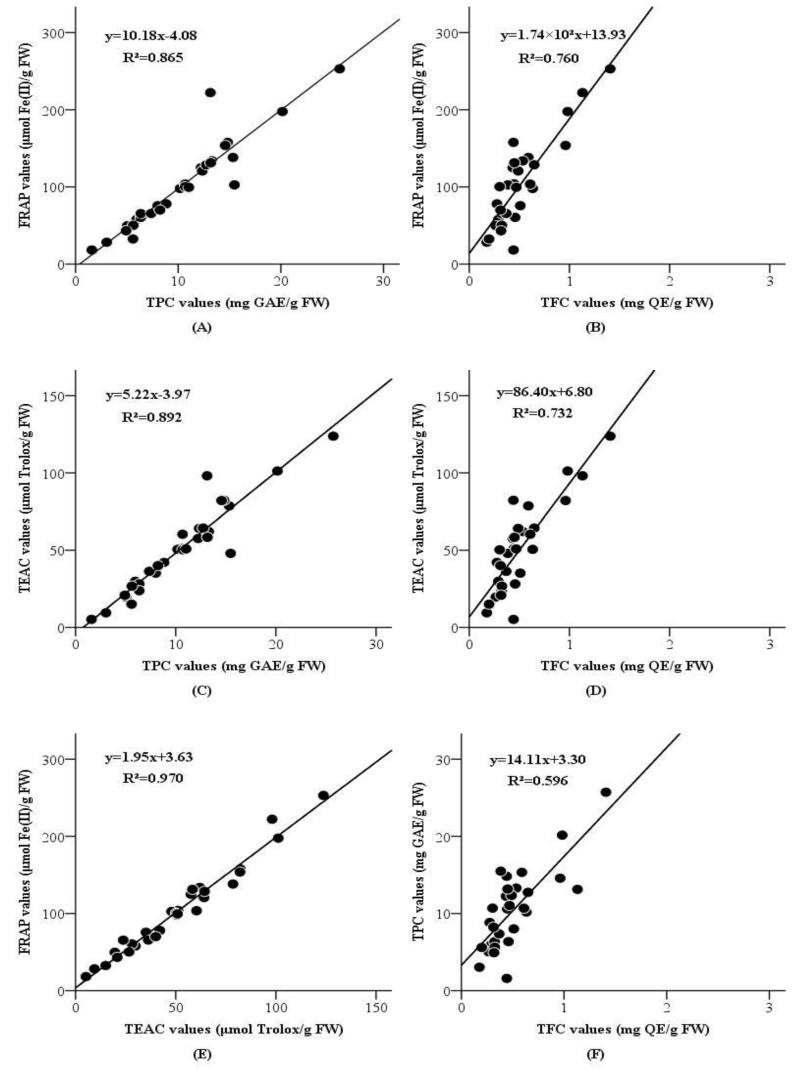
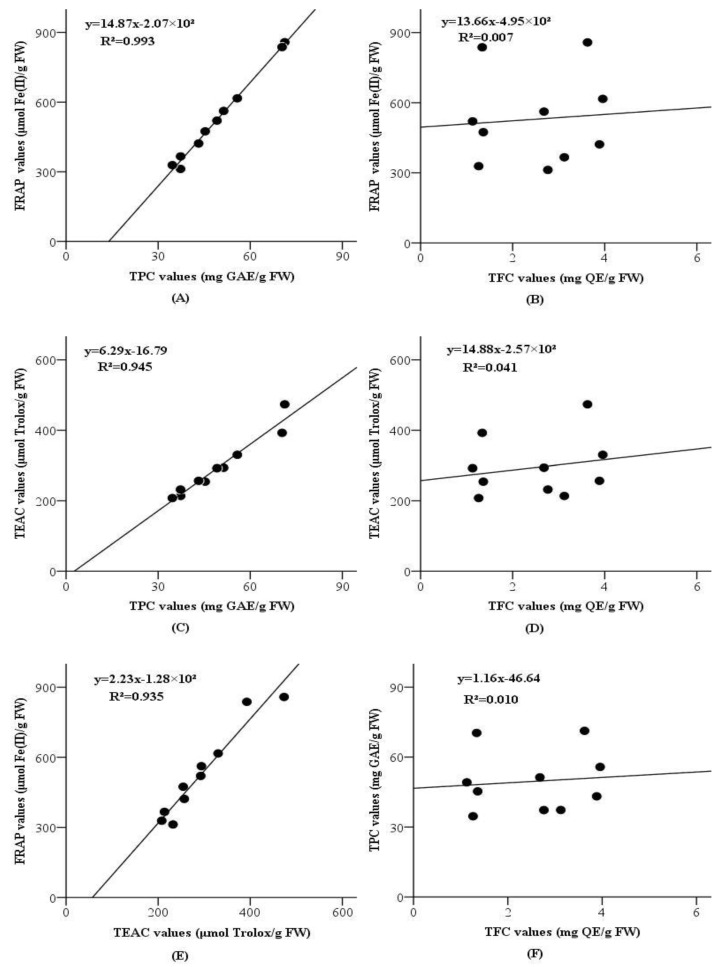
The correlations between FRAP, TEAC, TPC and TFC values (based on the total values of three fractions) were detected using a simple linear regression model, and the results were displayed in Figure 1 and Figure 2.
Figure 1.
Correlations between FRAP values and TPC values (A); FRAP values and TFC values (B); TEAC values and TPC values (C); TEAC values and TFC values (D); FRAP values and TEAC values (E); TPC values and TFC values (F) of peels from 30 grape varieties.
Figure 2.
Correlations between FRAP values and TPC values (A); FRAP values and TFC values (B); TEAC values and TPC values (C); TEAC values and TFC values (D); FRAP values and TEAC values (E); TPC values and TFC values (F) of seeds collected from 10 grape varieties.
For grape peels, as seen from Figure 1, FRAP values and TEAC values were highly correlated to TPC values (R² = 0.865, p < 0.001 and R² = 0.892, p < 0.001, respectively), and moderately correlated to TFC values (R² = 0.760, p < 0.001 and R² = 0.732, p < 0.001, respectively). The outcomes revealed that phenolic components could be the main ingredients responsible for the antioxidant capacities of the grape peels, and flavonoid compounds might contribute to the antioxidant capacities of grape peels but were not the main contributors. In addition, TPC values were weakly correlated with TFC values (R² = 0.596, p < 0.001). It suggested that flavonoids comprised only a small part of phenolic components of the grape peels. Furthermore, FRAP values were significantly correlated with TEAC values (R² = 0.970, p < 0.001), so the antioxidant ingredients in the grape peels could reduce oxidants (like Fe(III)) and scavenge free radicals (like ABTS•+).
For grape seeds, according to Figure 2, FRAP values and TEAC values were intensely correlated to TPC values (R² = 0.993, p < 0.001 and R² = 0.945, p < 0.001, respectively), but not correlated to TFC values (R² = 0.007, p = 0.825 and R² = 0.041, p = 0.574, respectively). The outcomes suggested that phenolic components could be the main contributors to the antioxidant capacities of the grape seeds, but flavonoid compounds had little influence on the antioxidant capacities of grape seeds. Additionally, there was no linear correlation between TPC values and TFC values (R² = 0.010, p = 0.779), which suggested that phenolic components of the grape seeds were rarely flavonoids. Moreover, the correlation between FRAP values and TEAC values was remarkable (R² = 0.935, p < 0.001), so the antioxidant components in these grape seeds could also reduce oxidants (like Fe(III)) and scavenge free radicals (like ABTS•+).
The results illustrated above are consistent with many previous studies, which have reported that phenolic components were the main contributors responsible for the antioxidant capacities, and could reduce oxidants and scavenge free radicals [31,33,34,35,36]. On the contrary, these results were quite different from some other studies that reported a very weak correlation (R2 = 0.0337) between the FRAP values and TEAC values [32,37], indicating that the ingredients possessing reducing activities and those possessing free radicals scavenging activities in the 62 fruits were not the same, and a very weak correlation (R2 = 0.0404) between the TEAC values and TPC values, suggesting that phenolic components could not be the main contributors to the free radicals scavenging abilities of the 62 fruits. Li et al. [37] also reported a very weak correlation between the TEAC values and the FRAP values (R2 = 0.1563) as well as the FRAP values and the TPC values (R2 = 0.1966), which suggested that phenolic components could not be the main contributors to activities of the 223 medicinal plants to reduce oxidants.
2.6. Phenolic Components of the Grape Peels and Seeds
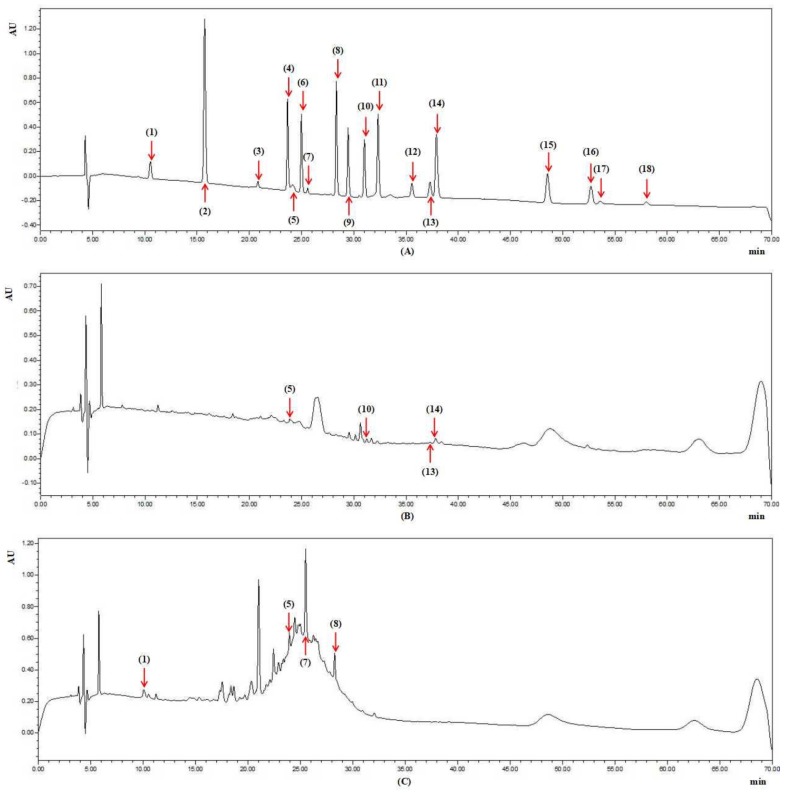
Phenolic components of the grape peels and seeds were determined on the base of the literature reported by Cai et al. with small alteration [54]. Phenolic components of the grape peels and seeds were detected, and the results were displayed in Table 5. Furthermore, the chromatograms under 220 nm of the mixed standards and the samples of Black Grape (Yunnan, China) peel and Pearl Black Grape (Xinjiang, China) seed were shown in Figure 3.
Table 5.
Phenolic components of peels and seeds from 30 grape varieties.
| Name of Grapes | Place of Production | Part of Grapes | Phenols | Total Contents (mg/g FW) |
|---|---|---|---|---|
| Black Grape | Yunnan, China | Peel | cyanidin-3-glucoside | 0.174 ± 0.009 |
| ferulaic acid | 0.241 ± 0.011 | |||
| rutin | 0.073 ± 0.006 | |||
| resveratrol | 0.266 ± 0.015 | |||
| Blackcurrant Grape | California, CA, USA | Peel | cyanidin-3-glucoside | 0.498 ± 0.028 |
| rutin | 0.687 ± 0.047 | |||
| Flame Grape | Xinjiang, China | Peel | cyanidin-3-glucoside | 0.421 ± 0.023 |
| ferulaic acid | 0.049 ± 0.003 | |||
| rutin | 0.367 ± 0.015 | |||
| Fragrant Green Grape | Yunnan, China | Peel | rutin | 0.383 ± 0.019 |
| Golden Finger Grape | California, CA, USA | Peel | cyanidin-3-glucoside | 0.150 ± 0.007 |
| ferulaic acid | 0.041 ± 0.003 | |||
| rutin | 0.569 ± 0.034 | |||
| Green Grape | Victoria, Australia | Peel | rutin | 0.268 ± 0.025 |
| Ito Kyoho Grape | Yunnan, China | Peel | epicatechin | 0.015 ± 0.001 |
| rutin | 0.035 ± 0.003 | |||
| Kyoho Grape | Liaoning, China | Peel | rutin | 0.113 ± 0.005 |
| Kyoho Grape | Xinjiang, China | Peel | epicatechin | 0.026 ± 0.002 |
| rutin | 0.129 ± 0.009 | |||
| Kyoho Grape | Guangxi, China | Peel | rutin | 0.138 ± 0.008 |
| Kyoho Grape | Yunnan, China | Peel | rutin | 0.117 ± 0.006 |
| Pearl Black Grape | Xinjiang, China | Peel | rutin | 0.199 ± 0.007 |
| Pearl Green Grape | Xinjiang, China | Peel | rutin | 0.016 ± 0.000 |
| Pearl Green Grape | Victoria, Australia | Peel | rutin | 0.047 ± 0.002 |
| Red Grape | Yunnan, China | Peel | cyanidin-3-glucoside | 0.326 ± 0.023 |
| rutin | 0.804 ± 0.055 | |||
| Red Grape | Guangxi, China | Peel | cyanidin-3-glucoside | 0.211 ± 0.007 |
| rutin | 0.293 ± 0.026 | |||
| Red Grape | Xinjiang, China | Peel | cyanidin-3-glucoside | 0.412 ± 0.033 |
| rutin | 0.298 ± 0.027 | |||
| Red Grape | California, China | Peel | cyanidin-3-glucoside | 0.377 ± 0.030 |
| rutin | 0.298 ± 0.020 | |||
| Rose Black Grape | Xinjiang, China | Peel | rutin | 0.137 ± 0.006 |
| Rose Black Grape | Yunnan, China | Peel | rutin | 0.030 ± 0.001 |
| Seedless Black Grape | Xinjiang, China | Peel | rutin | 0.059 ± 0.002 |
| Seedless Black Grape | California, CA, USA | Peel | rutin | 0.265 ± 0.022 |
| Seedless Dew Grape | Xinjiang, China | Peel | rutin | 0.049 ± 0.001 |
| Seedless Green Grape | Xinjiang, China | Peel | rutin | 0.008 ± 0.000 |
| Seedless Red Grape | Yunnan, China | Peel | rutin | 0.176 ± 0.012 |
| Seedless Red Grape | Xinjiang, China | Peel | cyanidin-3-glucoside | 0.021 ± 0.001 |
| rutin | 0.195 ± 0.013 | |||
| Seedless Red Grape | California, CA, USA | Peel | cyanidin-3-glucoside | 0.058 ± 0.003 |
| rutin | 0.666 ± 0.056 | |||
| Seedless Red Grape | Victoria, Australia | Peel | cyanidin-3-glucoside | 0.272 ± 0.011 |
| rutin | 0.594 ± 0.036 | |||
| Summer Black Grape | Shaanxi, China | Peel | epicatechin | 0.051 ± 0.004 |
| rutin | 0.150 ± 0.006 | |||
| Summer Black Grape | Xinjiang, China | Peel | rutin | 0.125 ± 0.003 |
| Black Grape | Yunnan, China | Seed | gallic acid | 0.146 ± 0.008 |
| cyanidin-3-glucoside | 0.305 ± 0.028 | |||
| epicatechin | 1.207 ± 0.074 | |||
| catechin gallate | 0.052 ± 0.002 | |||
| Ito Kyoho Grape | Yunnan, China | Seed | gallic acid | 0.054 ± 0.003 |
| cyanidin-3-glucoside | 0.840 ± 0.052 | |||
| epicatechin | 1.693 ± 0.094 | |||
| catechin gallate | 0.028 ± 0.002 | |||
| Kyoho Grape | Xinjiang, China | Seed | gallic acid | 0.066 ± 0.003 |
| cyanidin-3-glucoside | 0.180 ± 0.011 | |||
| epicatechin | 2.088 ± 0.106 | |||
| catechin gallate | 0.119 ± 0.004 | |||
| Kyoho Grape | Guangxi, China | Seed | gallic acid | 0.052 ± 0.002 |
| cyanidin-3-glucoside | 0.105 ± 0.005 | |||
| epicatechin | 2.039 ± 0.187 | |||
| catechin gallate | 0.044 ± 0.002 | |||
| Kyoho Grape | Yunnan, China | Seed | gallic acid | 0.087 ± 0.002 |
| cyanidin-3-glucoside | 0.202 ± 0.019 | |||
| epicatechin | 1.886 ± 0.165 | |||
| catechin gallate | 0.054 ± 0.002 | |||
| Pearl Black Grape | Xinjiang, China | Seed | gallic acid | 0.193 ± 0.017 |
| cyanidin-3-glucoside | 0.189 ± 0.009 | |||
| epicatechin | 1.745 ± 0.111 | |||
| catechin gallate | 0.126 ± 0.005 | |||
| Red Grape | Yunnan, China | Seed | gallic acid | 0.236 ± 0.009 |
| cyanidin-3-glucoside | 0.113 ± 0.003 | |||
| epicatechin | 2.156 ± 0.156 | |||
| catechin gallate | 0.176 ± 0.008 | |||
| Red Grape | Guangxi, China | Seed | gallic acid | 0.089 ± 0.004 |
| cyanidin-3-glucoside | 0.095 ± 0.005 | |||
| epicatechin | 1.547 ± 0.144 | |||
| catechin gallate | 0.145 ± 0.005 | |||
| Red Grape | Xinjiang, China | Seed | gallic acid | 0.056 ± 0.002 |
| cyanidin-3-glucoside | 0.058 ± 0.003 | |||
| epicatechin | 1.644 ± 0.098 | |||
| catechin gallate | 0.128 ± 0.004 | |||
| Red Grape | California, CA, USA | Seed | gallic acid | 0.022 ± 0.001 |
| cyanidin-3-glucoside | 0.111 ± 0.005 | |||
| epicatechin | 0.877 ± 0.065 | |||
| catechin gallate | 0.165 ± 0.013 |
Figure 3.
Chromatograms under 220 nm of the standard compounds (A); Black Grape (Yunnan, China) peel (B); Pearl Black Grape (Xinjiang, China) seed (C).The numbers in brackets refer to the compounds: gallic acid (1); protocatechuic acid (2); gallo catechin (3); chlorogenic acid (4); cyanidin-3-glucoside (5); caffeic acid (6); epicatechin (7); catechin gallate (8); p-coumaric acid (9); ferulaic acid (10); melatonin (11); 2-hydroxycinnamic acid (12); rutin (13); resveratrol (14); daidzein (15); equol (16); quercetin (17); genistein (18).
As seen from Table 5, five phenols, including cyanidin-3-glucoside, epicatechin, rutin, ferulaic acid and resveratrol, were found in the 30 grape peels. Every grape peel sample contained rutin, and the contents ranged from 0.008 ± 0.000 to 0.804 ± 0.055 mg/g FW with a 100-fold difference. The peel of Red Grape (Yunnan, China) possessed the highest level of rutin. Some grape peels contained cyanidin-3-glucoside, and the contents ranged from 0.021 ± 0.001 to 0.498 ± 0.028 mg/g FW with a 24-fold difference. The peel of Blackcurrant Grape (California, CA, USA) possessed the highest level of cyanidin-3-glucoside. The peels of Summer Black Grape (Shaanxi, China), Kyoho Grape (Xinjiang, China) and Ito Kyoho Grape (Yunnan, China) contained epicatechin of 0.051 ± 0.004, 0.026 ± 0.002 and 0.015 ± 0.001 mg/g FW, and the peels of Black Grape (Yunnan, China), Flame Grape (Xinjiang, China) and Golden Finger Grape (California, CA, USA) contained ferulaic acid of 0.241 ± 0.011, 0.049 ± 0.003 and 0.041 ± 0.003 mg/g FW, while resveratrol (0.266 ± 0.015 mg/g FW) was only detected in the peel of Black Grape (Yunnan, China).
As for the 10 grape seeds, four phenols including gallic acid, cyanidin-3-glucoside, epicatechin and catechin gallate were found in all of them, and the content ranges were as follows, respectively: 0.022 ± 0.001 to 0.236 ± 0.009 mg/g FW with a 10-fold difference; 0.058 ± 0.003 to 0.840 ± 0.052 mg/g FW with a 14-fold difference; 0.877 ± 0.065 to 2.156 ± 0.156 mg/g FW with a 2-fold difference; 0.028 ± 0.002 to 0.176 ± 0.008 mg/g FW with a 7-fold difference, respectively. The seeds of Red Grape (Yunnan, China), Ito Kyoho Grape (Yunnan, China), Red Grape (Yunnan, China) and Red Grape (Yunnan, China) possessed the highest level of gallic acid, cyanidin-3-glucoside, epicatechin and catechin gallate, respectively. These results also prove our hypothesis that grapes with diverse genotypes have different composition and contents of bioactive compounds. Furthermore, it was reported that phenols like cyanidin-3-glucoside, resveratrol and rutin possessed varies bioactivities, such as antibacterium, antioxidant, anti-inflammation and hepatic and cardiovascular protection, so grape peels and seeds from juice and wine industries could be valuable resources to extract phenols with further use in producing functional foods, food additives and pharmaceuticals.
The above results were expressed on the weight of fresh material. In addition, the moisture contents of the grape peels and seeds are displayed in Table 6, which could be used to express the results on the weight of dry material.
Table 6.
Moisture contents of the tested grape peels and seeds.
| Name of Grapes | Place of Production | Part of Grapes | Moisture Contents (%) |
|---|---|---|---|
| Black Grape | Yunnan, China | Peel | 72.333 ± 2.951 |
| Blackcurrant Grape | California, CA, USA | Peel | 72.382 ± 1.023 |
| Flame Grape | Xinjiang, China | Peel | 58.699 ± 2.487 |
| Fragrant Green Grape | Yunnan, China | Peel | 74.926 ± 3.156 |
| Golden Finger Grape | California, CA, USA | Peel | 71.851 ± 2.894 |
| Green Grape | Victoria, Australia | Peel | 79.027 ± 0.525 |
| Ito Kyoho Grape | Yunnan, China | Peel | 77.103 ± 3.446 |
| Kyoho Grape | Guangxi, China | Peel | 77.402 ± 1.568 |
| Kyoho Grape | Liaoning, China | Peel | 80.564 ± 3.699 |
| Kyoho Grape | Xinjiang, China | Peel | 78.757 ± 3.321 |
| Kyoho Grape | Yunnan, China | Peel | 79.920 ± 3.219 |
| Pearl Black Grape | Xinjiang, China | Peel | 79.023 ± 3.013 |
| Pearl Green Grape | Xinjiang, China | Peel | 74.881 ± 0.856 |
| Pearl Green Grape | Victoria, Australia | Peel | 75.233 ± 3.112 |
| Red Grape | California, CA, USA | Peel | 76.320 ± 3.239 |
| Red Grape | Guangxi, China | Peel | 77.416 ± 2.986 |
| Red Grape | Xinjiang, China | Peel | 73.806 ± 2.357 |
| Red Grape | Yunnan, China | Peel | 76.117 ± 2.766 |
| Rose Black Grape | Xinjiang, China | Peel | 69.404 ± 2.551 |
| Rose Black Grape | Yunnan, China | Peel | 69.726 ± 0.469 |
| Seedless Black Grape | California, CA, USA | Peel | 72.423 ± 2.995 |
| Seedless Black Grape | Xinjiang, China | Peel | 69.200 ± 2.210 |
| Seedless Dew Grape | Xinjiang, China | Peel | 74.168 ± 2.848 |
| Seedless Green Grape | Xinjiang, China | Peel | 72.232 ± 2.365 |
| Seedless Red Grape | California, CA, USA | Peel | 71.103 ± 1.334 |
| Seedless Red Grape | Victoria, Australia | Peel | 66.384 ± 2.085 |
| Seedless Red Grape | Xinjiang, China | Peel | 73.000 ± 2.462 |
| Seedless Red Grape | Yunnan, China | Peel | 73.293 ± 2.366 |
| Summer Black Grape | Shaanxi, China | Peel | 71.560 ± 2.232 |
| Summer Black Grape | Xinjiang, China | Peel | 69.450 ± 2.211 |
| Black Grape | Yunnan, China | Seed | 42.396 ± 1.845 |
| Ito Kyoho Grape | Yunnan, China | Seed | 44.002 ± 1.933 |
| Kyoho Grape | Xinjiang, China | Seed | 41.664 ± 0.759 |
| Kyoho Grape | Guangxi, China | Seed | 43.489 ± 1.926 |
| Kyoho Grape | Yunnan, China | Seed | 44.001 ± 1.988 |
| Pearl Black Grape | Xinjiang, China | Seed | 46.646 ± 2.003 |
| Red Grape | Yunnan, China | Seed | 52.859 ± 2.109 |
| Red Grape | Guangxi, China | Seed | 51.622 ± 0.221 |
| Red Grape | Xinjiang, China | Seed | 46.424 ± 1.903 |
| Red Grape | California, CA, USA | Seed | 51.570 ± 2.158 |
3. Materials and Methods
3.1. Chemical Reagents
The 2,2′-azinobis(3-ethylbenothiazoline-6-sulphonic acid) diammonium salt (ABTS), 6-hydroxy-2,5,7,8-tetramethylchromane-2-carboxylic acid (Trolox), 2,4,6-tri(2-pyridyl)-s-triazine (TPTZ), Folin-Ciocalteu’s phenol reagent, and the standard compounds (gallic acid, protocatechuic acid, gallo catechin, chlorogenic acid, cyanidin-3-glucoside, caffeic acid, epicatechin, catechin gallate, p-coumaric acid, ferulaic acid, melatonin, 2-hydroxycinnamic acid, rutin, resveratrol, daidzein, equol, quercetin and genistein) were provided by Sigma-Aldrich (St. Louis, MO, USA). Tetrahydrofuran, methanol, formic acid, diethyl ether and ethyl acetate were provided by Kermel Chemical Factory (Tianjin, China). Acetic acid, sodium acetate, potassium acetate, sodium hydroxide, hydrochloric acid, ethylenediaminetetraacetic acid (EDTA), ascorbic acid, iron (III) chloride hexahydrate (FeCl3·6H2O), iron(II) sulphate heptahydrate (FeSO4·7H2O), potassium persulphate, sodium carbonate, aluminum chloride hexahydrate (AlCl3·6H2O), ethanol and n-hexane were provided by Damao Chemical Factory (Tianjin, China). All chemical reagents used in the tests were of analytical or chromatographic grade, and the water used was double distilled.
3.2. Sample Preparation
Grapes from 30 varieties produced in China, USA and Australia (Figure 4) were obtained from local shops in Guangzhou, China. Grapes were washed with double distilled water and dried at room temperature. The grapes were separated into peels, pulps and seeds, and then the peels and seeds were respectively ground into particles using a special grinder for food processing. After that, accurate 2.000 g of the samples were weighed and extracted with 10 mL tetrahydrofuran at 30 °C for 30 min in a shaking water bath [55]. The samples were centrifuged at 4200 g for 10 min, and the supernatants were gathered. The extraction was repeated twice, and the supernatants were collected as the fat-soluble fractions. Subsequently, the residues were extracted with 10 mL methanol-acetic acid-water (50:3.7:46.3, v/v/v) mixture at 30 °C for 30 min in a shaking water bath, which was also repeated twice, and the supernatants were gathered up as the water-soluble fractions. Furthermore, the residues were hydrolyzed with 5 mL sodium hydroxide solution (2 mol/L NaOH, 10 mmol/L EDTA, 1% ascorbic acid) at 37 °C for 30 min in a shaking water bath, and then acidified to pH = 2 with 6 mol/L hydrochloric acid solution [56]. The mixtures were extracted twice with 5 mL n-hexane to eliminate fatty acids, which might be released during alkaline hydrolysis. Immediately, the mixtures were extracted twice with 5 mL diethyl ether and ethyl acetate mixture (1:1, v/v), and the organic phases were collected. The extracts were dried out at room temperature under a stream of N2 using an evaporator and reconstituted in ethanol as the insoluble-bound fractions. All extracts were preserved at −20 °C until tested.
Figure 4.
Coordinates of geographical areas of the tested grapes.
3.3. FRAP Assay
The FRAP assay was conducted referring to the literature with minor alterations [27]. Briefly, the FRAP reagent was a mixture of 300 mmol/L sodium acetate-acetic acid buffer (pH = 3.6), 10 mmol/L TPTZ solution (40 mmol/L hydrochloric acid solution as solvent) and 20 mmol/L FeCl3 solution (10:1:1, v/v/v), and it was prepared freshly and warmed to 37 °C in a water bath before used. The 0.1 mL properly diluted sample was combined with 3 mL FRAP reagent. After incubated at room temperature for 4 min, a CANY 722 visible spectrophotometer (Shanghai, China) was used to measure the absorbance of the mixtures at 593 nm. The size and volume of cuvette were 1 cm × 1 cm × 4.5 cm and 4.5 mL, respectively. The assay volumes were 1/2 to 2/3 of the volume of cuvette. The results were expressed as μmol Fe (II)/g FW of the grape peels or seeds.
3.4. TEAC Assay
The TEAC assay was carried out according to the literature with minor alterations [41]. Accordingly, the ABTS•+ stock solution was a mixture of 7 mmol/L ABTS solution and 2.45 mmol/L potassium persulphate solution (1:1, v/v), which was incubated in the dark for at least 16 h at room temperature and used within 2 days. The ABTS•+ working solution was obtained by diluting the stock solution with ethanol to an absorbance of 0.710 ± 0.05 at 734 nm. The samples were diluted approximately until they can inhibit 20–80% blank absorbance. Subsequently, the 0.1 mL properly diluted sample was mixed with 3.8 mL ABTS•+ working solution and measured at 734 nm after incubated at room temperature for 6 min. The percent of inhibition of absorbance was calculated to evaluate of the antioxidant capacity. The results were expressed as μmol Trolox/g FW of the grape peels or seeds.
3.5. Determination of TPC
The TPC values were determined based on procedures reported by Singleton, Orthofer and Lamuela-Raventos [49]. Briefly, a properly diluted sample (0.5 mL) was added to Folin-Ciocalteu reagent (0.2 mol/L, 2.5 mL). After 4 min, saturated sodium carbonate solution (about 75 g/L, 2 mL) was added to the mixture. The mixture was incubated at room temperature for 2 h, and then the absorbance was measured at 760 nm. The results were expressed as milligram gallic acid equivalent (mg GAE)/g FW of the grape peels or seeds.
3.6. Determination of TFC
The TFC values were determined according to the literature reported by Kalia et al. [50]. Accordingly, a properly diluted sample (0.5 mL) was mixed with ethanol solution (95%, v/v, 1.5 mL), AlCl3 solution (10%, w/v, 0.1 mL), potassium acetate solution (1 mol/L, 0.1 mL) and double distilled water (2.8 mL). The mixture was incubated for 30 min at room temperature, and then the absorbance was measured at 415 nm. The results were expressed as mg quercetin equivalent (mg QE)/g FW of the grape peels or seeds.
3.7. HPLC Analysis
The phenolic and flavonoid components in the samples were detected by HPLC-PDAD (photodiode array detector) based on the method reported by Cai et al. with small modifcations [54]. In detail, the HPLC system included a Waters (Milford, MA, USA) 1525 binary HPLC pump separation module with an auto-injector and employed a Waters 2996 PDAD. Separation was carried out with an Agilent Zorbax Extend-C18 column (250 × 4.6 mm, 5 μm) at 40 °C with a gradient elution solution A, composed of formic acid solution (0.1%, v/v), and solution B, methanol, which were routinely delivered at a flow rate of 0.8 mL/min according to the procedure: 0 min, 95% (A); 15 min, 80% (A); 20 min, 70% (A); 25 min, 63% (A); 40 min, 60% (A); 60 min, 50% (A); 65 min, 50% (A); 65.1 min, 95% (A); and 70 min, 95% (A). Fat-soluble, water-soluble and insoluble-bound fractions were combined together before sampling. The spectra were recorded between 200 and 600 nm to characterize the peak patterns. Phenolic and flavonoid components were identified by the retention time and UV-Vis spectra comparing with standards and quantified by the peak area under maximum absorption wavelength, and the results were expressed as mg/g FW of the grape peels or seeds.
3.8. Data Analysis
All tests were conducted in triplicate and the values were expressed as mean ± SD (standard deviation). Data analysis was performed using SPSS 22 (International Business Machines Corporation, Armonk, NY, USA) and Excel 2007 (Microsoft Corporation, Redmond, WA, USA).
4. Conclusions
In this study, the antioxidant capacities and total phenolic and flavonoid contents of peels and seeds from 30 grape varieties were systematically evaluated. The antioxidant capacities and phenolic and flavonoid contents of the grape peels and seeds were greatly different, and those of the three fractions were, in decreasing order: fat-soluble fractions > water-soluble fractions > insoluble-bound fractions. Antioxidant components in these grape peels and seeds could reduce oxidants and scavenge free radicals, and phenols were the main contributors to the antioxidant capacities, and flavonoids were not major contributors to these activities. Several phenolic compounds such as gallic acid, cyanidin-3-glucoside, epicatechin, catechin gallate, ferulaic acid, rutin and resveratrol were identified and quantified in these grape peels and seeds. These grape wastes could be abundant sources of natural bioactive compounds for developing functional foods, food additives and pharmaceuticals.
Acknowledgments
We acknowledge Dr. Qin Xiao for technical support.
Author Contributions
Conceptualization, G.-Y.T., S.L. and H.-B.L.; Methodology, G.-Y.T. and C.-N.Z.; Software, Q.L.; Validation, G.-Y.T.; Formal Analysis, C.-N.Z.; Investigation, G.-Y.T., C.-N.Z., Q.L., X.-L.F., X.-Y.X., S.-Y.C. and X.M.; Resources, G.-Y.T.; Data Curation, C.-N.Z.; Writing-Original Draft Preparation, G.-Y.T.; Writing-Review & Editing, S.L., R.-Y.G. and H.-B.L.; Visualization, G.-Y.T.; Supervision, H.-B.L.; Project Administration, H.-B.L.; Funding Acquisition, R.-Y.G. and H.-B.L.
Funding
This work was supported by the Shanghai Pujiang Talent Plan (No. 18PJ1404600); Shanghai Basic and Key Program (No. 18JC1410800); National Natural Science Foundation of China (No. 81372976); Key Project of Guangdong Provincial Science and Technology Program (No. 2014B020205002); and the Hundred-Talents Scheme of Sun Yat-Sen University.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of the compounds are unavailable from the authors.
References
- 1.Patel S. Grape seeds: Agro-industrial waste with vast functional food potential. In: Patel S., editor. Emerging Bioresources with Nutraceutical and Pharmaceutical Prospects. Volume 3. Springer International Publishing AG; Cham, Switzerland: 2015. pp. 53–69. [Google Scholar]
- 2.Clifton P.M. Effect of grape seed extract and quercetin on cardiovascular and endothelial parameters in high-risk subjects. J. Biomed. Biotech. 2004;2004:272–278. doi: 10.1155/S1110724304403088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hosu A., Cristea V., Cimpoiu C. Analysis of total phenolic, flavonoids, anthocyanins and tannins content in Romanian red wines: Prediction of antioxidant activities and classification of wines using artificial neural networks. Food Chem. 2014;150:113–118. doi: 10.1016/j.foodchem.2013.10.153. [DOI] [PubMed] [Google Scholar]
- 4.Park M., Cho H., Jung H., Lee H., Hwang K.T. Antioxidant and anti-inflammatory activities of tannin fraction of the extract from black raspberry seeds compared to grape seeds. J. Food Biochem. 2014;38:259–270. doi: 10.1111/jfbc.12044. [DOI] [Google Scholar]
- 5.Dillenburg D.R., Mostarda C., Moraes-Silva I.C., Ferreira D., Goncalves Bos D.D.S., Machado Duarte A.A., Irigoyen M.C., Rigatto K. Resveratrol and grape juice differentially ameliorate cardiovascular autonomic modulation in L-NAME-treated rats. Auton. Neurosci.-Basic. 2013;179:9–13. doi: 10.1016/j.autneu.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 6.Tome-Carneiro J., Gonzalvez M., Larrosa M., Garcia-Almagro F.J., Aviles-Plaza F., Parra S., Yanez-Gascon M.J., Ruiz-Ros J.A., Garcia-Conesa M.T., Tomas-Barberan F.A., et al. Consumption of a grape extract supplement containing resveratrol decreases oxidized LDL and ApoB in patients undergoing primary prevention of cardiovascular disease: A triple-blind, 6-month follow-up, placebo-controlled, randomized trial. Mol. Nutr. Food Res. 2012;56:810–821. doi: 10.1002/mnfr.201100673. [DOI] [PubMed] [Google Scholar]
- 7.Vanzo A., Terdoslavich M., Brandoni A., Torres A.M., Vrhovsek U., Passamonti S. Uptake of grape anthocyanins into the rat kidney and the involvement of bilitranslocase. Mol. Nutr. Food Res. 2008;52:1106–1116. doi: 10.1002/mnfr.200700505. [DOI] [PubMed] [Google Scholar]
- 8.Cadiz-Gurrea M.D., Borras-Linares I., Lozano-Sanchez J., Joven J., Fernandez-Arroyo S., Segura-Carretero A. Cocoa and grape seed byproducts as a source of antioxidant and anti-inflammatory proanthocyanidins. Int. J. Mol. Sci. 2017;18:376. doi: 10.3390/ijms18020376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu C., Yagiz Y., Hsu W., Simonne A., Lu J., Marshall M.R. Antioxidant, antibacterial, and antibiofilm properties of polyphenols from muscadine grape (Vitis rotundifolia Michx.) pomace against selected foodborne pathogens. J. Agric. Food Chem. 2014;62:6640–6649. doi: 10.1021/jf501073q. [DOI] [PubMed] [Google Scholar]
- 10.Iannone M., Mare R., Paolino D., Gagliardi A., Froiio F., Cosco D., Fresta M. Characterization and in vitro anticancer properties of chitosan-microencapsulated flavan-3-ols-rich grape seed extracts. Int. J. Biol. Macromol. 2017;104:1039–1045. doi: 10.1016/j.ijbiomac.2017.07.022. [DOI] [PubMed] [Google Scholar]
- 11.Ferri M., Rondini G., Calabretta M.M., Michelini E., Vallini V., Fava F., Roda A., Minnucci G., Tassoni A. White grape pomace extracts, obtained by a sequential enzymatic plus ethanol-based extraction, exert antioxidant, anti-tyrosinase and anti-inflammatory activities. New Biotechnol. 2017;39:51–58. doi: 10.1016/j.nbt.2017.07.002. [DOI] [PubMed] [Google Scholar]
- 12.Vaisman N., Niv E. Daily consumption of red grape cell powder in a dietary dose improves cardiovascular parameters: A. double blind, placebo-controlled, randomized study. Int. J. Food Sci. Nutr. 2015;66:342–349. doi: 10.3109/09637486.2014.1000840. [DOI] [PubMed] [Google Scholar]
- 13.Ismail A., Salem A., Eassawy M. Hepatoprotective effect of grape seed oil against carbon tetrachloride induced oxidative stress in liver of gamma-irradiated rat. J. Photoch. Photobio. B. 2016;160:1–10. doi: 10.1016/j.jphotobiol.2016.03.027. [DOI] [PubMed] [Google Scholar]
- 14.Tang G.Y., Meng X., Li Y., Zhao C.N., Liu Q., Li H.B. Effects of vegetables on cardiovascular diseases and related mechanisms. Nutrients. 2017;9:857. doi: 10.3390/nu9080857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhao C.N., Meng X., Li Y., Li S., Liu Q., Tang G.Y., Li H.B. Fruits for prevention and treatment of cardiovascular diseases. Nutrients. 2017;9:598. doi: 10.3390/nu9060598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meng X., Li Y., Li S., Gan R.Y., Li H.B. Natural products for prevention and treatment of chemical-induced liver injuries. Compr. Rev. Food Sci. Food Saf. 2018;17:472–495. doi: 10.1111/1541-4337.12335. [DOI] [PubMed] [Google Scholar]
- 17.Li Y., Li S., Meng X., Gan R.Y., Zhang J.J., Li H.B. Dietary natural products for prevention and treatment of breast cancer. Nutrients. 2017;9:728. doi: 10.3390/nu9070728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wittenauer J., Schweiggert-Weisz U., Carle R. In vitro-study of antioxidant extracts from Garcinia mangostana pericarp and Riesling grape pomace-a contribution to by-products valorization as cosmetic ingredients. J. Appl. Bot. Food Qual. 2016;89:249–257. [Google Scholar]
- 19.Sadovoy V.V., Shchedrina T.V., Shlykov S.N., Trubina I.A., Salimov M.A. Antioxidant food additive of the red grapes berry peel. Pishchevaya Promyshlennost. 2013:68–70. [Google Scholar]
- 20.Xia E.Q., Deng G.F., Guo Y.J., Li H.B. Biological activities of polyphenols from grapes. Int. J. Mol. Sci. 2010;11:622–646. doi: 10.3390/ijms11020622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Percival S.S., West R.L. Effect of health-promoting properties of grapes, including resveratrol. In: Skinner M., Hunter D., editors. Bioactives in Fruit: Health Benefits and Functional Foods. Blackwell Science Publ.; Oxford, UK: 2013. pp. 197–214. [Google Scholar]
- 22.Meral R., Dogan I.S. Grape seed as a functional food ingredient in bread-making. Int. J. Food Sci. Nutr. 2013;64:372–379. doi: 10.3109/09637486.2012.738650. [DOI] [PubMed] [Google Scholar]
- 23.Ananga A., Georgiev V., Ochieng J., Phills B., Tsolova V. Production of anthocyanins in grape cell cultures: A potential source of raw material for pharmaceutical, food, and cosmetic industries. In: Poljuha D., Sladonja B., editors. Mediterranean Genetic Code-Grapevine and Olive. Intech. Europe; Rijeka, Croatia: 2013. pp. 247–287. [Google Scholar]
- 24.Michalska A., Wojdylo A., Lysiak G.P., Figiel A. Chemical composition and antioxidant properties of powders obtained from different plum juice formulations. Int. J. Mol. Sci. 2017;18:176. doi: 10.3390/ijms18010176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.He J.Z., Xu Y.Y., Chen H.B., Sun P.L. Extraction, structural characterization, and potential antioxidant activity of the polysaccharides from four seaweeds. Int. J. Mol. Sci. 2016;17:1988. doi: 10.3390/ijms17121988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Garrido I., Uriarte D., Hernandez M., Llerena J.L., Valdes M.E., Espinosa F. The evolution of total phenolic compounds and antioxidant activities during ripening of grapes (Vitis vinifera L., cv. Tempranillo) grown in semiarid region: Effects of cluster thinning and water deficit. Int. J. Mol. Sci. 2016;17:1923. doi: 10.3390/ijms17111923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Benzie I.F.F., Strain J.J. The ferric reducing ability of plasma (FRAP) as a measure of “Antioxidant power”: The FRAP assay. Anal. Biochem. 1996;239:70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- 28.Raudonis R., Raudone L., Jakstas V., Janulis V. Comparative evaluation of post-column free radical scavenging and ferric reducing antioxidant power assays for screening of antioxidants in strawberries. J. Chromatogr. A. 2012;1233:8–15. doi: 10.1016/j.chroma.2012.02.019. [DOI] [PubMed] [Google Scholar]
- 29.Hayes W.A., Mills D.S., Neville R.F., Kiddie J., Collins L.M. Determination of the molar extinction coefficient for the ferric reducing/antioxidant power assay. Anal. Biochem. 2011;416:202–205. doi: 10.1016/j.ab.2011.05.031. [DOI] [PubMed] [Google Scholar]
- 30.Pohanka M., Bandouchova H., Sobotka J., Sedlackova J., Soukupova I., Pikula J. Ferric reducing antioxidant power and square wave voltammetry for assay of low molecular weight antioxidants in blood plasma: Performance and comparison of methods. Sensors-Basel. 2009;9:9094–9103. doi: 10.3390/s91109094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Deng G.F., Lin X., Xu X.R., Gao L.L., Xie J.F., Li H.B. Antioxidant capacities and total phenolic contents of 56 vegetables. J. Funct. Foods. 2013;5:260–266. doi: 10.1016/j.jff.2012.10.015. [DOI] [Google Scholar]
- 32.Fu L., Xu B.T., Xu X.R., Gan R.Y., Zhang Y., Xia E.Q., Li H.B. Antioxidant capacities and total phenolic contents of 62 fruits. Food Chem. 2011;129:345–350. doi: 10.1016/j.foodchem.2011.04.079. [DOI] [PubMed] [Google Scholar]
- 33.Guo Y.J., Deng G.F., Xu X.R., Wu S., Li S., Xia E.Q., Li F., Chen F., Ling W.H., Li H.B. Antioxidant capacities, phenolic compounds and polysaccharide contents of 49 edible macro-fungi. Food Funct. 2012;3:1195–1205. doi: 10.1039/c2fo30110e. [DOI] [PubMed] [Google Scholar]
- 34.Deng G., Shen C., Xu X.R., Kuang R.D., Guo Y.J., Zeng L., Gao L.L., Lin X., Xie J.F., Xia E.Q., et al. Potential of fruit wastes as natural resources of bioactive compounds. Int. J. Mol. Sci. 2012;13:8308–8323. doi: 10.3390/ijms13078308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fu L., Xu B.T., Xu X.R., Qin X.S., Gan R.Y., Li H.B. Antioxidant capacities and total phenolic contents of 56 wild fruits from South China. Molecules. 2010;15:8602–8617. doi: 10.3390/molecules15128602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li A.N., Li S., Li H.B., Xu D.P., Xu X.R., Chen F. Total phenolic contents and antioxidant capacities of 51 edible and wild flowers. J. Funct. Foods. 2014;6:319–330. doi: 10.1016/j.jff.2013.10.022. [DOI] [Google Scholar]
- 37.Li S., Li S.K., Gan R.Y., Song F.L., Kuang L., Li H.B. Antioxidant capacities and total phenolic contents of infusions from 223 medicinal plants. Ind. Crop. Prod. 2013;51:289–298. doi: 10.1016/j.indcrop.2013.09.017. [DOI] [Google Scholar]
- 38.Nilsson J., Pillai D., Onning G., Persson C., Nilsson A., Akesson B. Comparison of the 2,2′-azinobis-3-ethylbenzotiazoline-6-sulfonic acid (ABTS) and ferric reducing antioxidant power (FRAP) methods to asses the total antioxidant capacity in extracts of fruit and vegetables. Mol. Nutr. Food Res. 2005;49:239–246. doi: 10.1002/mnfr.200400083. [DOI] [PubMed] [Google Scholar]
- 39.Van den Berg R., Haenen G., van den Berg H., van der Vijgh W., Bast A. The predictive value of the antioxidant capacity of structurally related flavonoids using the Trolox equivalent antioxidant capacity (TEAC) assay. Food Chem. 2000;70:391–395. doi: 10.1016/S0308-8146(00)00092-3. [DOI] [Google Scholar]
- 40.Van den Berg R., Haenen G., van den Berg H., Bast A. Applicability of an improved Trolox equivalent antioxidant capacity (TEAC) assay for evaluation of antioxidant capacity measurements of mixtures. Food Chem. 1999;66:511–517. doi: 10.1016/S0308-8146(99)00089-8. [DOI] [Google Scholar]
- 41.Re R., Pellegrini N., Proteggente A., Pannala A., Yang M., Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radical Bio. Med. 1999;26:1231–1237. doi: 10.1016/S0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- 42.Badami S., Moorkoth S., Rai S.R., Kannan E., Bhojraj S. Antioxidant activity of Caesalpinia sappan heartwood. Biol. Pharm. Bull. 2003;26:1534–1537. doi: 10.1248/bpb.26.1534. [DOI] [PubMed] [Google Scholar]
- 43.Murcia M.A., Martinez-Tome M., Jimenez A.M., Vera A.M., Honrubia M., Parras P. Antioxidant activity of edible fungi (truffles and mushrooms): Losses during industrial processing. J. Food Protect. 2002;65:1614–1622. doi: 10.4315/0362-028X-65.10.1614. [DOI] [PubMed] [Google Scholar]
- 44.Deng G.F., Xu X.R., Guo Y.J., Xia E.Q., Li S., Wu S., Chen F., Ling W.H., Li H.B. Determination of antioxidant property and their lipophilic and hydrophilic phenolic contents in cereal grains. J. Funct. Foods. 2012;4:906–914. doi: 10.1016/j.jff.2012.06.008. [DOI] [Google Scholar]
- 45.Dorsey T.E., McDonald P.W., Roels O.A. A heated Biuret-Folin protein assay which gives equal absorbance with different proteins. Anal. Biochem. 1977;78:156–164. doi: 10.1016/0003-2697(77)90019-7. [DOI] [PubMed] [Google Scholar]
- 46.Vinson J.A., Proch J., Bose P. Determination of quantity and quality of polyphenol antioxidants in foods and beverages. Meth. Enzymol. 2001;35:103–114. doi: 10.1016/s0076-6879(01)35235-7. [DOI] [PubMed] [Google Scholar]
- 47.Chun O.K., Kim D.O. Consideration on equivalent chemicals in total phenolic assay of chlorogenic acid-rich plums. Food Res. Int. 2004;37:337–342. doi: 10.1016/j.foodres.2004.02.001. [DOI] [Google Scholar]
- 48.Roura E., Andres-Lacueva C., Estruch R., Lamucla-Raventos R.M. Total polyphenol intake estimated by a modified Folin-Ciocalteu assay of urine. Clin. Chem. 2006;52:749–752. doi: 10.1373/clinchem.2005.063628. [DOI] [PubMed] [Google Scholar]
- 49.Singleton V.L., Orthofer R., Lamuela-Raventos R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. In: Packer L., editor. Oxidants and Antioxidants, PTA. Volume 299. Elsevier Academic Press Inc.; San Diego, CA, USA: 1999. pp. 152–178. [Google Scholar]
- 50.Kalia K., Sharma K., Singh H.P., Singh B. Effects of extraction methods on phenolic contents and antioxidant activity in aerial parts of Potentilla atrosanguinea lodd. and quantification of its phenolic constituents by RP-HPLC. J. Agric. Food Chem. 2008;56:10129–10134. doi: 10.1021/jf802188b. [DOI] [PubMed] [Google Scholar]
- 51.Teixeira T.S., Vale R.C., Almeida R.R., Ferreira T.P.S., Guimaraes G.L. Antioxidant potential and its correlation with the contents of phenolic compounds and flavonoids of methanolic extracts from different medicinal plants. Revista Virtual De Quimica. 2017;9:1546–1559. doi: 10.21577/1984-6835.20170090. [DOI] [Google Scholar]
- 52.Kuppusamy S., Thavamani P., Megharaj M., Nirola R., Lee Y.B., Naidu R. Assessment of antioxidant activity, minerals, phenols and flavonoid contents of common plant/tree waste extracts. Ind. Crop. Prod. 2016;83:630–634. doi: 10.1016/j.indcrop.2015.12.060. [DOI] [Google Scholar]
- 53.Gan R., Wang M., Lui W., Wu K., Dai S., Sui Z., Corke H. Diversity in antioxidant capacity, phenolic contents, and flavonoid contents of 42 edible beans from China. Cereal Chem. 2017;94:291–297. doi: 10.1094/CCHEM-03-16-0061-R. [DOI] [Google Scholar]
- 54.Cai Y.Z., Luo Q., Sun M., Corke H. Antioxidant activity and phenolic compounds of 112 traditional Chinese medicinal plants associated with anticancer. Life Sci. 2004;74:2157–2184. doi: 10.1016/j.lfs.2003.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li L., Chen C.Y.O., Chun H., Cho S., Park K., Lee-Kim Y.C., Blumberg J.B., Russell R.M., Yeum K. A fluorometric assay to determine antioxidant activity of both hydrophilic and lipophilic components in plant foods. J. Nutr. Biochem. 2009;20:219–226. doi: 10.1016/j.jnutbio.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 56.Nardini M., Cirillo E., Natella F., Mencarelli D., Comisso A., Scaccini C. Detection of bound phenolic acids: Prevention by ascorbic acid and ethylenediaminetetraacetic acid of degradation of phenolic acids during alkaline hydrolysis. Food Chem. 2002;79:119–124. doi: 10.1016/S0308-8146(02)00213-3. [DOI] [Google Scholar]