Table 1.
Chemical structure of evaluated compounds 1a–p, their surface tension γ (relative surface activity; in N/m units), wavelengths of observed absorption maxima (λ1, λ2 (Ch-T), λ3) and logarithms of molar absorption coefficients (log ε1, log ε2 (Ch-T), log ε3) of compounds’ methanolic solutions (c = 8.0 × 10−5 M), which were investigated in the UV/Vis region of an electromagnetic spectrum.
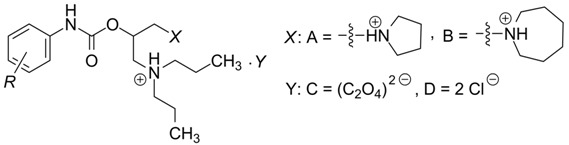
| ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Comp. | R | X | Y | γ (N/m) | λ 1 | log ε1 | λ 2 (Ch-T) | 1 log ε2 (Ch-T) | λ 3 | log ε3 |
| 1a | 2-OC4H9 | A | C | 0.06464 | 208 | 4.51 | 236 | 4.19 | 280 | 3.63 |
| 1b | 2-OC5H11 | A | C | 0.06366 | 208 | 4.39 | 236 | 4.05 | 280 | 3.51 |
| 1c | 2-OC6H13 | A | C | 0.06222 | 208 | 4.58 | 238 | 4.52 | 278 | 3.49 |
| 1d | 2-OC7H15 | A | C | 0.05985 | 208 | 4.43 | 236 | 4.08 | 280 | 3.55 |
| 1e | 3-OC4H9 | A | D | 0.06316 | 210 | 4.65 | 238 | 4.24 | 279 | 3.59 |
| 1f | 3-OC5H11 | A | D | 0.06285 | 210 | 4.55 | 237 | 4.27 | 279 | 3.62 |
| 1g | 3-OC6H13 | A | D | 0.06105 | 210 | 4.54 | 237 | 4.13 | 279 | 3.48 |
| 1h | 3-OC7H15 | A | D | 0.05786 | 210 | 4.66 | 237 | 4.27 | 279 | 3.63 |
| 1i | 2-OC4H9 | B | C | 0.06302 | 208 | 4.44 | 236 | 4.08 | 280 | 3.57 |
| 1j | 2-OC5H11 | B | C | 0.06206 | 208 | 4.54 | 236 | 4.22 | 280 | 3.71 |
| 1k | 2-OC6H13 | B | C | 0.06065 | 208 | 4.42 | 236 | 4.10 | 280 | 3.57 |
| 1l | 2-OC7H15 | B | C | 0.05853 | 208 | 4.44 | 236 | 4.14 | 280 | 3.59 |
| 1m | 3-OC4H9 | B | D | 0.06298 | 210 | 4.52 | 238 | 4.09 | 279 | 3.44 |
| 1n | 3-OC5H11 | B | D | 0.06154 | 210 | 4.56 | 238 | 4.18 | 279 | 3.52 |
| 1o | 3-OC6H13 | B | D | 0.05925 | 210 | 4.52 | 238 | 4.01 | 279 | 3.37 |
| 1p | 3-OC7H15 | B | D | 0.05692 | 210 | 4.65 | 238 | 4.20 | 279 | 3.56 |
1 log ε2 (Ch-T), Logarithms of molar absorption coefficients observed at the charge-transfer absorption maximum λ2 (Ch-T) = 236–238 nm.
