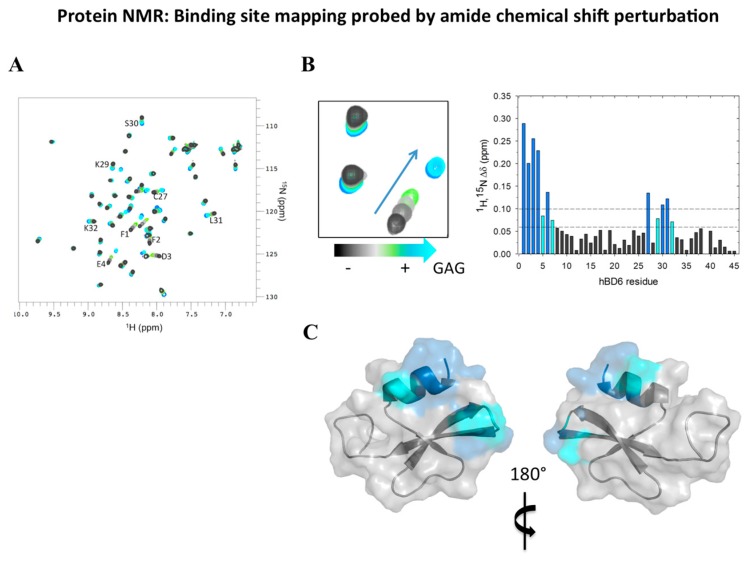
Figure 4.
Nuclear magnetic resonance (NMR) as a tool to determine ligand-defensin complex formation. (A) Once the backbone chemical shift assignment is accomplished, a 1H-15N heteronuclear single quantum coherence (HSQC) spectrum of the free protein containing the assigned amide groups is obtained. (B) Titration of an unlabeled ligand (in this example, a glycosaminoglycan [GAG]) bound to 15N-labeled human β-defensin (HBD)6 results in a chemical shift perturbation map of HBD6-ligand interactions. The NMR titration revealed large changes in the chemical shift of HBD6 in a fast-exchange equilibrium between free and GAG-bound HBD6, as demonstrated by a gradual shift in peak positions in 1H-15N HSQC spectra. (C) The amide groups significantly perturbed upon GAG binding are highlighted on the HBD6 surface (protein data bank: 2LWL) in blue and cyan to map the binding sites for the GAG. Figure adapted from [41]. For further details see Section 2.5.