Table 4.
Substrate scope of N-sulfonyl imine 2 and N-alkylsulfonamide 3 formation.
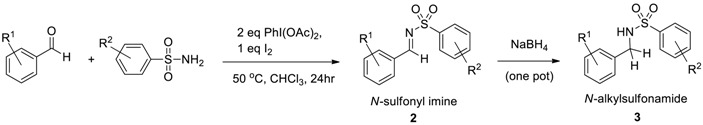
| Entry | (Aldehyde) R1 | (Sulfonamide) R2 | Product 2 Yield (%) 1 | Product 3 Yield (%) 2 |
|---|---|---|---|---|
| 1 | 4-OCH3 | 4-CH3 | 2aa 61 | 3aa 61 |
| 2 | 4-CH3 | 4-CH3 | 2ba 71 | 3ba 60 |
| 3 | H | 4-CH3 | 2ca 67 | 3ca 50 |
| 4 | 4-Br | 4-CH3 | 2da 54 | 3da 45 |
| 5 | 4-Cl | 4-CH3 | 2ea 65 | 3ea 74 |
| 6 | 4-NO2 | 4-CH3 | 2fa 50 | 3fa 40 |
| 7 | 4-CN | 4-CH3 | 2ga 52 | 3ga 44 |
| 8 | 3-CH3 | 4-CH3 | 2ha 70 | 3ha 33 |
| 9 | 2-CH3 | 4-CH3 | 2ia 60 | 3ia 33 |
| 10 | 3-OCH3 | 4-CH3 | 2ja 60 | 3ja 40 |
| 11 | 2-OCH3 | 4-CH3 | 2ka 65 | 3ka 40 |
| 12 | 3-Br | 4-CH3 | 2la 50 | 3la 22 |
| 13 | 2-Br | 4-CH3 | 2ma 36 | 3ma 30 |
| 14 | 4-CH3 | H | 2bb 69 | 3bb 40 |
| 15 | 4-Cl | H | 2eb 66 | 3eb 70 |
| 16 | 2-CH3 | H | 2hb 60 | 3hb 38 |
| 17 | 4-CH3 | 4-Cl | 2bc 60 | 3bc 40 |
| 18 | 4-Br | 4-Cl | 2dc 58 | 3dc 54 |
| 19 | 4-CH3 | 2-Cl | 2bd 35 | 3bd 17 |
| 20 | 4-OCH3 | 4-NO2 | 2ae 34 | 3ae 11 |
| 21 | 4-Cl | 4-NO2 | 2ee 27 | 3ee 21 |
| 22 | 4-CH3 | 3-NO2 | 2bf 25 | 3bf 8 |
| 23 | 4-CH3 | 2-NO2 | 2bg 6 | 3bg trace |
1 Yields are based upon 1H-NMR integration with internal standard. 2 Isolated yields.
