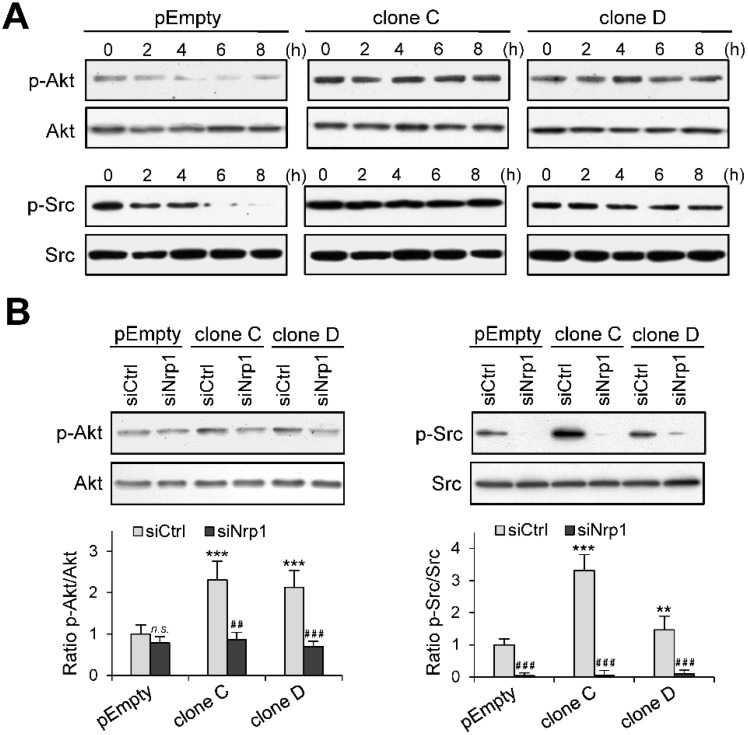
Figure 6.
Effect of Nrp1 silencing on HS3ST3B-mediated activation of Akt and Src kinases. Parental (pEmpty) and HS3ST3B expressing (clones C and D) cells were transfected with siRNA targeting Nrp1 (siNrp1) or negative siRNA (siCtrl) and then cultured for 24 h in complete medium. (A) After wash, cells were cultured in the absence of FCS for 8 h. Every two h, cells were collected and lysed. Proteins were then separated by SDS-PAGE and subjected to Western blotting with antibodies to the phosphorylated forms of Akt and Src. Parallel immunoblotting with antibodies to Akt and Src regardless of their phosphorylation status confirmed equal loading of samples. Representative results from three independent experiments are shown. (B) Parental and HS3ST3B expressing (clones C and D) cells were transfected with siRNAs as above. After serum starvation for 2 h, the phosphorylated forms of Akt and Src was detected in cell lysates by Western blot. Parallel immunoblotting with anti-Akt and anti-Src antibodies confirmed equal loading of the samples. Representative results from three independent experiments are shown. Histograms represent the quantification of the phosphorylation status of Akt and Src. Data were normalized to the parental cells that have been transfected with siCtrl (** p < 0.01, *** p < 0.001, significantly different when compared with the parental cells; ## p < 0.01, ### p < 0.001, significantly different when compared with the siCtrl-treated cells; n.s., not significant).