Abstract
Background
Higher fibroblast growth factor‐23 (FGF‐23) levels are associated with incident heart failure (HF) in MESA (the Multiethnic Study of Atherosclerosis). FGF‐23 is also associated with left ventricular hypertrophy. Whether the FGF‐23 association with HF is similar for heart failure with reduced ejection fraction (HFrEF) and heart failure with preserved ejection fraction (HFpEF) is not well established.
Methods and Results
We studied 6542 participants (mean age 62±10 years, 53% women, mean estimated glomerular filtration rate of 81±18 mL/min per 73 m2) from MESA who were free of cardiovascular disease at baseline (2000–2002). HF events were ascertained by an adjudication committee for a median follow‐up of 12.1 years. We classified HF events as HFrEF (ejection fraction [EF] <50%) or HFpEF [EF] ≥50%) at the time of diagnosis. Cox proportional hazard regression was used to compute hazard ratios and 95% confidence intervals for the association between baseline serum FGF‐23 and incident HFrEF and HFpEF. A total of 134 events were classified as HFpEF, 151 HFrEF, and 49 unknown EF. Following imputation, 149 were classified as HFpEF, 176 HFrEF, and 291 participants had HF (34 participants had HFpEF then HFrEF). In the fully adjusted model, higher FGF‐23 levels were associated with incident HFpEF but not with HFrEF (hazard ratio 1.29, 95% confidence interval, 1.08–1.54) versus (hazard ratio 1.04, 95% confidence interval, 0.84–1.29) for each 20 pg/mL higher serum FGF‐23 concentration.
Conclusions
FGF‐23 association with HF is driven by the association with HFpEF but not with HFrEF in a population‐based cohort. Further studies are needed to determine the pathological mechanisms mediating this association.
Keywords: ejection fraction, fibroblast growth factor, heart failure, left ventricular hypertrophy
Subject Categories: Heart Failure, Hypertrophy, Growth Factors/Cytokines, Cardiomyopathy, Biomarkers
Clinical Perspective
What Is New?
Fibroblast growth factor‐23 is a new novel biomarker for heart failure with preserved ejection fraction independent of traditional risk factors.
What Are the Clinical Implications?
Further studies are needed to determine whether changes in fibroblast growth factor‐23 levels will affect heart failure with preserved ejection fraction incidence and management.
Introduction
Heart failure (HF) is a major cause of morbidity and mortality and associated high healthcare‐related costs.1 It is estimated to affect almost 5.7 million Americans and >23 million people worldwide.2, 3 Half of these people have normal left ventricular ejection fraction (EF) >50% and are classified as having HF with preserved ejection fraction (HFpEF).4 While the morbidity and mortality of HF with reduced ejection fraction (HFrEF) is improving,5 outcomes for HFpEF are unchanged.6, 7, 8
Although several evidence‐based therapies are now available for the treatment of patients with HFrEF,9 the same cannot be said about HFpEF.10 In fact, the exact pathophysiologic processes, which are considered to be multifactorial and heterogeneous, are not completely understood.11, 12, 13 Additional research is needed to better characterize the phenotype of HFpEF, and identify risk factors and pathophysiological processes associated with this condition.
Fibroblast growth factor‐23 (FGF‐23), a hormone involved in phosphorus and vitamin D homeostasis,14, 15, 16 is linked to the development of HF and left ventricular hypertrophy (LVH).17, 18 The administration of recombinant FGF‐23 promotes cardiomyocyte growth in animal models and higher circulating FGF‐23 concentrations are associated with HF and cardiovascular events in kidney disease and general populations.18 Among 6542 participants in MESA (Multiethnic Study of Atherosclerosis), higher FGF‐23 concentrations were associated with all incident HF events over 7.5 years of follow‐up.19 Such associations could represent HFpEF, given known biological effects of FGF‐23 on ventricular hypertrophy. On the other hand, LVH is associated with HFrEF, and FGF‐23 is associated with other pathologies that could promote HFrEF, such as arterial stiffness, endothelial dysfunction, and vascular calcifications.20
The primary goal of this study is to delineate and contrast associations of serum FGF‐23 concentrations with incident HFpEF and HFrEF events in MESA, a multiethnic community‐based cohort that was free of cardiovascular disease at baseline and includes adjudicated HF events during long‐term follow‐up. In a supplemental analysis among participants with cardiac magnetic resonance (CMR) imaging at baseline, we also tested whether these associations were explained in part by differences in left ventricular (LV) mass.
Methods
The data, analytic methods, and study materials will be/have been made available to other researchers for purposes of reproducing the results or replicating the procedure. The MESA mechanism for public access to data is via the BioLINCC repository.21
Study Population
MESA is a prospective cohort study of cardiovascular disease among 6814 community‐living individuals free of clinical cardiovascular disease and aged 45 to 84 years of age at enrollment.21 Between 2000 and 2002, individuals were recruited from 6 US sites: Baltimore, MD; Chicago, IL; St. Paul, MN; Forsyth County, NC; New York, NY; and Los Angeles, CA. MESA recruited a final population that was 38% white, 28% black, 22% Hispanic, and 12% Asian, predominantly of Chinese descent. The study excluded people with self‐reported history of myocardial infarction, angina, stroke, transient ischemic attack, heart failure, atrial fibrillation, nitroglycerin use, angioplasty, coronary artery bypass grafting, valve replacement, pacemaker or defibrillator, or any surgery on the heart or arteries. All participants gave informed consent, and institutional review board approval was obtained for each site.
We evaluated all participants who had serum FGF‐23 measurements at baseline and who were followed for the development of either incident HFrEF or HFpEF through 2012 (N=6542; Figure 1). Three participants were excluded because they had extremely out of range FGF‐23 values and were considered by the laboratory to represent laboratory error.
Figure 1.
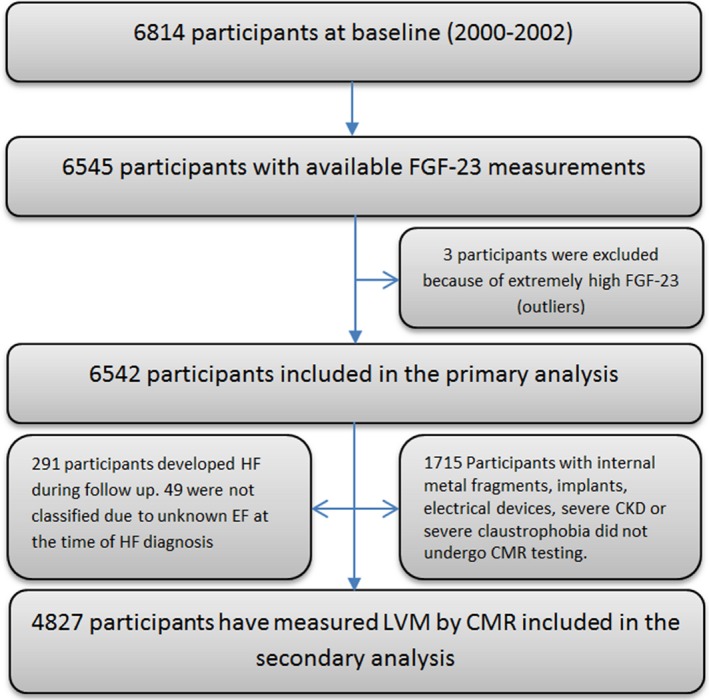
Participant enrollment for the current study. CKD indicates chronic kidney disease; CMR, cardiac magnetic resonance; EF, ejection fraction; FGF‐23, fibroblast growth factor‐23; HF, heart failure; LVM, left ventricular mass.
Measurements of Other Variables
Medical and personal histories were ascertained using standardized questionnaires. Information was gathered to ascertain participant's demographic data, tobacco usage, alcohol consumption, medical conditions, and current use of prescription and nonprescription medications and supplements. Blood pressure, ECGs, and laboratory data were obtained as previously described.21 Body mass index was calculated as weight in kilograms divided by height in meters squared. MESA investigators defined diabetes mellitus by the use of a diabetes mellitus medication or a fasting blood glucose level ≥126 mg/dL. Hypertension was defined as systolic blood pressure ≥140 mm Hg and/or diastolic blood pressure ≥90 mm Hg or medical treatment for hypertension.
The University of Vermont Laboratory for Clinical Biochemistry measured serum creatinine using a modified Jaffe reaction that was indirectly calibrated to Cleveland Clinic laboratory standards, serum cystatin C and C‐reactive protein concentrations using a BNII system nephelometer (Siemens), and urine albumin and creatinine from spot morning collections using nephelometry and the rate Jaffe reaction, respectively. Serum and urine phosphate were measured from frozen samples using timed‐rate colorimetry on a Beckman‐Coulter DxC chemistry analyzer. Estimated glomerular filtration rate (eGFR) was calculated using serum creatinine and cystatin C concentrations from the 2012 CKD‐EPI equation.22
Study Procedures: CMR Imaging
A subset of MESA participants (n=4980) without contraindications (internal metal fragments, implants, electrical devices, or severe claustrophobia) underwent CMR imaging at the MESA baseline examination by using 1.5‐T scanners (Avanto and Espree, Siemens Medical Systems, Erlangen, Germany) with a 6‐channel anterior phased‐array torso coil and corresponding posterior coil elements. LV function, dimensions, and myocardial mass were assessed by a cine steady‐state free precession sequence. Twelve short‐axis slices, one 4‐chamber view, and one 2‐chamber view were acquired as previously described.23 The endocardial and epicardial borders were contoured using a semiautomated method. The difference between the endocardial and epicardial areas for all slices was multiplied by slice thickness and section cap and then multiplied by the specific myocardial density (1.04 g/mL) to determine LV mass.
Measurement of Serum FGF‐23 Concentrations
Blood samples stored at the University of Vermont Laboratory for Clinical Biochemistry were shipped on dry ice to the University of Washington, where serum FGF‐23 concentrations were measured using the Kainos Immunoassay,24 which detects the full‐length biologically intact FGF‐23 molecule via midmolecule and distal epitopes. Standardized high‐ and low‐value FGF‐23 controls within each run were used to monitor quality control. The coefficient of variation for singlicate high and low control samples across 81 plates were 6.7% and 12.4%, respectively.
Ascertainment of HF Events
MESA personnel screened participants for incident clinical events through telephone contacts and scheduled follow‐up examinations. Personnel abstracted any hospital records suggesting cardiovascular events and recorded symptoms, history, and biomarkers; scanned ECGs, echocardiograms, catheterization reports, outpatient records, and other relevant diagnostic reports; and transmitted these to the Coordinating Center.
Two study physicians blinded to other study data independently reviewed the medical records. Incident HF events were considered as probable or definite. Definite or probable HF required HF symptoms, such as shortness of breath or edema, as asymptomatic disease is not a MESA end point. In addition to symptoms, probable HF required HF diagnosed by a physician and patient receiving medical treatment for HF. Definite HF required 1 or more other criteria, including pulmonary edema/congestion by chest radiograph; dilated ventricle or poor LV function by echocardiography or ventriculography; or evidence of LV diastolic dysfunction. We considered participants with a physician diagnosis of HF but without any other HF criteria as having no HF. The Events Committee classified HF as HFrEF (EF <50%) or HFpEF (EF≥50%) at the time of HF diagnosis. The EF was obtained from echocardiographic or radionuclide data available at the HF diagnosis (Figure 1).
Before imputation, 134 were classified as HFpEF, 151 as HFrEF, and 49 HF events were not classified. Sixteen participants had 1 type and then had the other type. The imputation was done for each HF type separately and therefore was not mutually exclusive. Following imputation, 149 events were classified as HFpEF and 176 as HFrEF (149+176=325). Thirty‐four participants had 1 type of HF and then had the other (eg, HFpEF then HFrEF), therefore they were only included once for HF (325–34=291). Thus, a total of 291 participants had HF during follow‐up.
Statistical Analysis
Continuous variables are presented as mean±SD and categorical variables as frequencies and percentages. Baseline characteristics were compared across serum FGF‐23 quartiles. We explored the functional relationship of serum FGF‐23 concentrations with HF events using restricted cubic spline models; given linear associations for each HF type, we proceeded to analyze FGF‐23 as a continuous linear variable, scaled to increments of 20 pg/mL to facilitate interpretation. We further evaluated the association between FGF‐23 and each HF type using quartiles of FGF‐23 to ensure that a threshold effect does not exist. We evaluated the time to first HFrEF and HFpEF event using separate Cox proportion hazards models for each HF type without censoring on the opposing type to avoid potential bias. Unknown HF types (N=49) were handled through multiple imputation using chained equations that were combined using Rubin's rules to account for variability in the imputation procedure. All covariates from the fully adjusted model were used in the imputation.25, 26 Models were adjusted as follows; Model 1 was unadjusted. Model 2 was adjusted for age, sex, race/ethnicity, highest education level completed (high school or less, some college but no degree, college degree or more), study site, height, and weight. Model 3 was further adjusted for systolic blood pressure (continuous), antihypertensive medications (yes; no), diabetes mellitus status (yes; no), high‐sensitivity C‐reactive protein, smoking (current versus former and never), urine albumin–creatinine ratio, and eGFRCKD‐Epi. Model 4 was further adjusted for N‐terminal pro B‐type natriuretic peptide, 25(OH)vitamin D, parathyroid hormone, and phosphate. We tested for the proportional hazards assumption and found no evidence of departure from this assumption.
In sensitivity analyses, we adjusted for LV mass detected by CMR to estimate the amount of association that may be explained through this characteristic. Furthermore, we used time‐updated covariates for additional sensitivity analyses using the following variables as time‐dependent covariates: height, weight, diabetes mellitus, smoking, urine albumin–creatinine ratio, and eGFRCKD‐Epi. In final sensitivity analysis, we also looked at complete case analysis by HF type. A 2‐sided P value of <0.05 was considered statistically significant for all analyses including interaction terms. Statistical analyses were performed using SAS software, version 9.3 (SAS Institute, Cary, NC), and R 3.3.2 (R Core Team; 2016, Vienna, Austria), and SPSS 24.0 (IBM Corp., 2016, Armonk, NY).
Results
There were 6542 participants (4827 of whom had CMR data) included in our analysis (Figure 1). Participants (mean age 62±10 years) included 53% women, 39% white, 12% Chinese, 27% black, and 22% Hispanic. The mean serum FGF‐23 concentration was 40±15 pg/mL and mean eGFR was 81±18 mL/min per 1.73 m2. Compared with participants in the lowest FGF‐23 quartile (<31 pg/mL), those in the highest quartile (>46 pg/mL) were older, had higher body mass index, higher systolic blood pressure and LV mass, lower eGFR, and were more likely to have diabetes mellitus (Table 1).
Table 1.
Baseline Characteristics by FGF‐23 Quartiles: The Multiethnic Study of Atherosclerosis 2000–2002
| Characteristic | Overall | FGF‐23 Quartiles | P Value | |||
|---|---|---|---|---|---|---|
| <31 pg/mL | 31–38 pg/mL | 38–46 pg/mL | >46 pg/mL | |||
| No. of participants | 6542 | 1636 | 1635 | 1636 | 1635 | |
| Age, y | 62±10 | 61±10 | 62±10 | 62±10 | 64±10 | <0.001 |
| Sex | ||||||
| Men | 3058 (47%) | 678 (41%) | 786 (48%) | 783 (48%) | 811 (50%) | <0.001 |
| Race/ethnicity | ||||||
| White, % | 2539 (39%) | 524 (32%) | 621 (38%) | 655 (40%) | 739 (45%) | <0.001 |
| Chinese, % | 795 (12%) | 192 (12%) | 214 (13%) | 194 (12%) | 195 (12%) | |
| Black, % | 1777 (27%) | 485 (30%) | 444 (27%) | 432 (26%) | 416 (25%) | |
| Hispanic, % | 1431 (22%) | 435 (27%) | 356 (22%) | 355 (22%) | 285 (17%) | |
| Diabetes mellitus | 809 (12%) | 203 (12%) | 201 (12%) | 175 (11%) | 230 (14%) | 0.03 |
| Current smoking | 843 (13%) | 274 (17%) | 218 (13%) | 199 (12%) | 152 (9%) | <0.001 |
| Education | ||||||
| High school or less (%) | 2314 (36%) | 527 (32%) | 606 (37%) | 576 (35%) | 605 (37%) | 0.004 |
| Some college (%) | 1174 (18%) | 333 (21%) | 324 (20%) | 289 (18%) | 228 (14%) | |
| College degree or more (%) | 3032 (46%) | 769 (47%) | 701 (43%) | 764 (47%) | 798 (49%) | |
| Body mass index (kg/m2) | 28±5.5 | 27.8±5.4 | 27.9±5.2 | 28.5±5.4 | 29±5.6 | <0.001 |
| SBP, mm Hg | 126±22 | 125±22 | 125±21 | 126±21 | 129±22 | <0.001 |
| HTN medication | 2400 (37%) | 511 (31%) | 534 (33%) | 583 (36%) | 772 (47%) | <0.001 |
| Estimated GFR, mL/min per 1.73 m2 | 81±18 | 87±17 | 83±20 | 81±17 | 75±18 | <0.001 |
| LV mass (g) (n=4827) | 120±29 | 116±28 | 120±29 | 121±30 | 124±31 | <0.001 |
Values are mean±SD. FGF‐23 indicates fibroblast growth factor‐23; GFR, glomerular filtration rate; HTN, hypertension; SBP, systolic blood pressure; Smoking, current and former vs never; LV mass, left ventricular mass.
During a median follow‐up of 12.1 (interquartile range: 11.6–12.7) years, 291 participants developed incident HF. Following imputation, 176 were classified as HFrEF and 149 were classified as HFpEF. Qualitatively, higher serum FGF‐23 concentrations corresponded with progressively greater risks of HFpEF in a generally linear pattern (Figure 2). However, there was no graphical evidence for a relationship of serum FGF‐23 concentrations with HFrEF events. Incidence rates reported as 1000‐person‐years for both HFrEF and HFpEF by FGF‐23 quartiles are shown (Figure 3).
Figure 2.
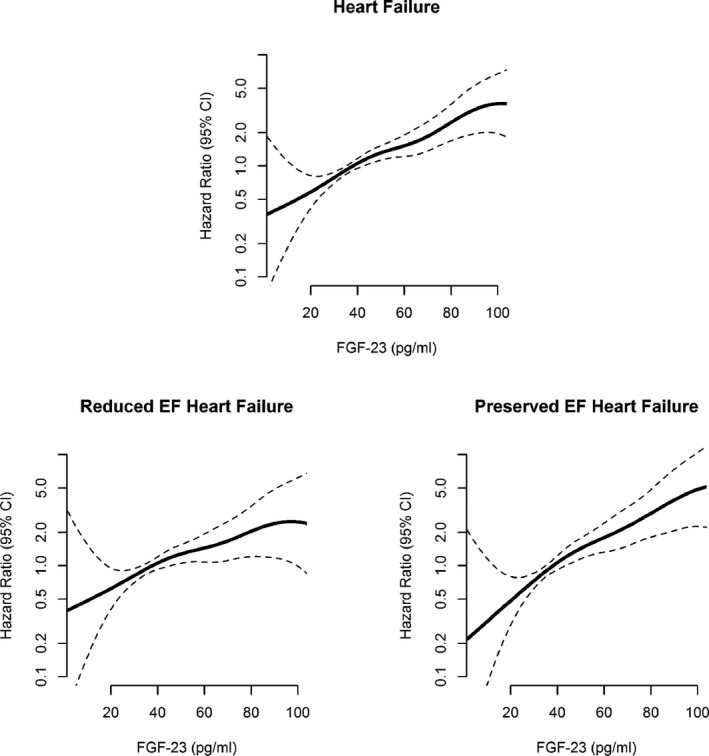
Hazard ratios of FGF‐23 for incident HF, HFrEF, and HFpEF using restricted cubic spline Cox model analysis: The Multiethnic Study of Atherosclerosis. CI indicates confidence interval; FGF‐23, fibroblast growth factor‐23; HF, heart failure; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction.
Figure 3.
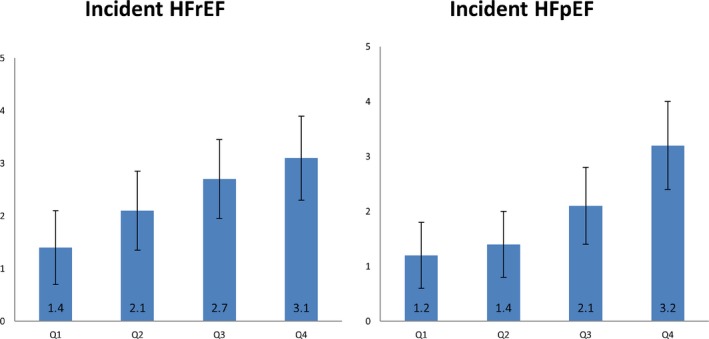
Incidence rates (per 1000 person‐years) by FGF‐23 quartiles of incident HFrEF and HFpEF: The Multiethnic Study of Atherosclerosis. FGF‐23 indicates fibroblast growth factor‐23; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction.
In unadjusted models, higher serum FGF‐23 concentrations were associated with a greater hazard of HFpEF events. After full adjustment for demographics, comorbidities, medications, and socioeconomic variables, each 20 pg/mL higher FGF‐23 concentration was associated with an estimated 29% greater risk of incident HFpEF (hazard ratio [HR] 1.29; 95% confidence interval, 1.08–1.54, P value 0.001) (Table 2). In contrast, serum FGF‐23 concentrations were not associated with HFrEF events in fully adjusted analyses. In quartiles analyses, the risk of incident HF was significantly higher in the highest quartile compared with the lowest quartile of FGF‐23 (HR 1.51; 95% confidence interval, 1.00–2.30) (Table 2).While similar results were observed for HFpEF with higher FGF‐23 quartiles (HR 1.85; 95% confidence interval, 1.03–3.33), there was no significant association with HFrEF (HR 1.25; 95% confidence interval, 0.72–2.17). There was a trend toward an association of FGF‐23 with unclassified HF events that was attenuated in fully adjusted models (Table S1).
Table 2.
Associations of FGF‐23 and Incident HF, HFrEF, and HFpEF (HRs for Each 20 pg/mL Higher FGF‐23 Concentration and Per Quartiles of FGF‐23): The Multiethnic Study of Atherosclerosis
| Participants (n) | Events (n) | Model 1 | Model 2 | Model 3 | Model 4 | |
|---|---|---|---|---|---|---|
| HR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) | |||
| Incident HF | ||||||
| FGF‐23 | ||||||
| per 20 pg/L increase | 6542 | 291 | 1.34 (1.21, 1.48) | 1.20 (1.08, 1.35) | 1.17 (1.04, 1.32) | 1.18 (1.02, 1.37) |
| Q1 | 1638 | 45 | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) |
| Q2 | 1636 | 62 | 1.30 (0.84, 2.01) | 1.14 (0.74, 1.77) | 1.18 (0.76, 1.83) | 1.17 (0.75, 1.80) |
| Q3 | 1634 | 81 | 1.67 (1.11, 2.52) | 1.34 (0.88, 2.03) | 1.45 (0.95, 2.21) | 1.37 (0.90, 2.08) |
| Q4 | 1634 | 103 | 2.44 (1.66, 3.61) | 1.72 (1.15, 2.56) | 1.68 (1.11, 2.54) | 1.51 (1.00, 2.30) |
| Incident HFrEF | ||||||
| FGF‐23 | ||||||
| per 20 pg/L increase | 6542 | 176 | 1.33 (1.13, 1.56) | 1.25 (1.04, 1.51) | 1.13 (0.93, 1.37) | 1.04 (0.84, 1.29) |
| Q1 | 1638 | 27 | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) |
| Q2 | 1636 | 40 | 1.40 (0.81, 2.42) | 1.26 (0.73, 2.18) | 1.32 (0.76, 2.30) | 1.29 (0.75, 2.24) |
| Q3 | 1634 | 51 | 1.56 (0.92, 2.66) | 1.31 (0.76, 2.24) | 1.40 (0.81, 2.42) | 1.26 (0.73, 2.17) |
| Q4 | 1634 | 58 | 2.22 (1.34, 3.67) | 1.65 (0.98, 2.77) | 1.50 (0.88, 2.57) | 1.25 (0.72, 2.17) |
| Incident HFpEF | ||||||
| FGF‐23 | ||||||
| per 20 pg/L increase | 6542 | 149 | 1.48 (1.28, 1.70) | 1.36 (1.15, 1.61) | 1.31 (1.11, 1.55) | 1.29 (1.08, 1.54) |
| Q1 | 1638 | 23 | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) |
| Q2 | 1636 | 27 | 1.17 (0.61, 2.24) | 1.01 (0.53, 1.94) | 1.06 (0.55, 2.04) | 1.05 (0.55, 2.02) |
| Q3 | 1634 | 40 | 1.90 (1.06, 3.41) | 1.46 (0.81, 2.64) | 1.59 (0.87, 2.89) | 1.51 (0.83, 2.75) |
| Q4 | 1634 | 59 | 3.00 (1.73, 5.21) | 1.96 (1.12, 3.44) | 1.99 (1.11, 3.57) | 1.85 (1.03, 3.33) |
Cox proportional models were used to calculate the HRs for the development of incident HF, HFrEF, and HFpEF with each 20 pg/mL higher serum FGF‐23 concentration and again with quartiles of FGF‐23. Model 1; unadjusted, Model 2; adjusted for age, sex, race/ethnicity, education, study site, height, and weight; Model 3 adjusted for Model 2 and systolic blood pressure, antihypertensive medications, diabetes mellitus, smoking, C‐reactive protein, urine albumin–creatinine ratio, and eGFRCKD‐EPI. Model 4 is further adjusted for NT‐proBNP, 25(OH) vitamin D, PTH, and phosphate. CI indicates confidence interval; eGFR, glomerular filtration rate; FGF‐23, fibroblast growth factor‐23; HF, heart failure; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; HR, hazard ratio; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; PTH, parathyroid hormone.
In sensitivity analyses, we evaluated the association between serum FGF‐23 and HFpEF for the subset of participants with measured LV mass at baseline (N=4827) after adjustment for Model 4 used in the main analysis, then after additional adjustment for LV mass (Table S2). Greater FGF‐23 concentrations (per 20 pg/mL higher) still remained statistically significantly associated with HFpEF risk even after accounting for LV mass (HR 1.28, 95% confidence interval, 1.02–1.62).
Additionally, we observed similar results using time‐updated covariates (Table S3).
In final sensitivity analysis looking at the complete case analysis by HF type, we found similar results (Table S4).
Discussion
In summary, we found higher serum FGF‐23 concentrations to be associated with HFpEF events, but not HFrEF events, in a large, multiethnic population that was initially free of clinical cardiovascular disease. Associations were observed for adjudicated HF events and HF type over long‐term follow‐up and included adjustment for relevant comorbid conditions and other potential confounding characteristics. Associations of FGF‐23 and HFpEF remained significant even after accounting for differences in LV mass. These findings suggest that FGF‐23 is a novel risk factor for HFpEF in the general population.
FGF‐23, a phosphaturic hormone that inhibits 1,25(OH)2 (activated) vitamin D production, has been associated with a wide range of cardiovascular outcomes, particularly among patients who have chronic kidney disease. Specific associations have been reported for incident hypertension, LVH, incident HF, higher coronary calcium scores, and incident coronary heart disease.17, 19, 27, 28 In this study we confirmed previous findings reported by Kestenbaum et al linking serum FGF‐23 to incident HF, and further studied the association with subtypes of HFpEF and HFrEF.
Potential mechanisms by which FGF‐23 could be linked to incident HFpEF include FGF‐23 effects on LVH, cardiac remodeling, neurohormonal changes, sodium retention, and myocardial fibrosis. In experimental models, administration of recombinant FGF‐23 to mice affects cardiac myocytes directly and induces LVH.18, 29 FGF‐23 exerts a direct stimulatory effect on the renin‐angiotensin‐aldosterone system through suppression of angiotensin‐converting enzyme‐2,30 which suggests an alternative mechanism of the negative cardiovascular outcomes associated with FGF‐23. Other mechanisms by which FGF‐23 could lead to adverse cardiovascular consequences include calcitriol deficiency, altered phosphorus homeostasis, systemic inflammation and oxidative stress, and sodium retention.31, 32, 33 Additionally, FGF‐23 may downregulate soluble α‐klotho,34 an enzyme with anti‐aging effects that was shown to protect the heart against cardiac hypertrophy and cardiac remodeling.35, 36 In a study by Reindl et al, FGF‐23 was significantly higher in patients who developed LV remodeling post ST‐segment–elevation myocardial infarction even after adjustment for biomarkers of myocardial necrosis, myocardial stress, and inflammation.37 Finally, FGF‐23 may promote myocardial fibrosis through upregulation of β‐catenin, transforming growth factor‐β, procollagen I, and procollagen III, which may exacerbate diastolic dysfunction.38
Several previous reports demonstrated that higher LV mass is associated with greater risk of HF events.39, 40, 41 In a study by Velagaleti et al, eccentric LVH was associated with higher risk of HFrEF, while concentric LVH was associated with higher risk of HFpEF.39 Furthermore, Seliger et al demonstrated that LVH was associated with higher risk of both types of HF, but especially HFrEF in older adults without prior HF or myocardial infarction in the Cardiovascular Health Study.41 Our findings of a strong association between higher FGF‐23 and higher risk of HFpEF cannot be explained fully by previous observations of the association between FGF‐23 and LV mass.18, 19 In sensitivity analyses we found that the association of FGF‐23 and HF remained significant even after adjusting for LV mass, suggesting that other pathological mechanisms could be mediating the association between FGF‐23 and the development of HF as mentioned in previous paragraphs. Nonetheless, LV mass measurements were performed on only a single occasion; it remains possible that changes in LV mass could have contributed to the observed association.
In this study, we found no significant association between higher FGF‐23 and HFrEF events, while previous studies suggested an association of higher FGF‐23 concentrations with lower LVEF.42, 43, 44 However, these studies showed very modest association with reduced EF. For example, in a study by Agarwal et al, the difference in mean EF between the lowest and highest quartiles of FGF‐23 was negligible (62.2% versus 59.7%, respectively) and was still considered as normal EF.42 Furthermore, in a post–kidney transplant cohort, no significant association was found between FGF‐23 and LVEF.45 Further longitudinal studies are needed to study the significance of these observations.
Several previous studies showed important associations between N‐terminal pro B‐type natriuretic peptide and incident HF,46 atrial fibrillation,47 and subclinical cardiovascular disease.48 Ndumele et al demonstrated that N‐terminal pro B‐type natriuretic peptide is a strong predictor of incident HF in individuals with and without obesity.46 We demonstrated in this study that FGF‐23 is significantly associated with incident HFpEF independent of N‐terminal pro B‐type natriuretic peptide levels, which suggests that FGF‐23 may be a novel predictor of incident HFpEF in the general population.
To our knowledge, this is the first study comparing the associations of FGF‐23 with HFrEF and HFpEF. Previous studies examined the association between FGF‐23 with EF as a continuous variable. Kestenbaum et al found no significant relationship between FGF‐23 and EF.19 In previous community‐based studies, FGF‐23 was associated with reduced EF.44, 49 However, these studies were in participants with chronic kidney disease,49 and in a population undergoing elective coronary angiography.44 Both of these populations have sicker participants with more cardiovascular risk factors.
Our study has several strengths, including the large, multiethnic, community‐based population, which improves the external validity of the study findings. MESA participants were free of clinical cardiovascular disease at baseline, reducing the possibility of confounding by this condition. Participants were followed for more than 10 years for ascertainment of relatively large numbers of incident HF events, which were adjudicated by an expert panel.
Our study has some limitations. FGF‐23 is an individual biomarker within complex pathways of bone and phosphate metabolism. These pathways include other factors that are linked with FGF‐23 and could also influence HF development. Furthermore, as we mentioned in the Methods section, diagnosis of HFpEF is sometimes difficult and requires physical examination, assessment of LVEF, LV diastolic function, chest radiograph, and other supportive findings, all of which are subject to potential errors. Thus, potential misclassification of these events cannot be excluded. In some instances, the type of HF could not be determined, reducing study power and potentially inducing misclassification. Finally, relatively few MESA participants had overtly low eGFR values, precluding analyses of associations in a CKD population, in which FGF‐23 concentrations are substantially higher. Furthermore, no causal relationships between FGF‐23 and HFpEF can be established.
In summary, higher serum FGF‐23 concentrations are associated with higher risk of incident HFpEF but not with HFrEF in the MESA.
Sources of Funding
This research was supported by grant R01‐HL‐096875. MESA is supported by contracts HHSN268201500003I, N01‐HC‐95159, N01‐HC‐95160, N01‐HC‐95161, N01‐HC‐95162, N01‐HC‐95163, N01‐HC‐95164, N01‐HC‐95165, N01‐HC‐95166, N01‐HC‐95167, N01‐HC‐95168, and N01‐HC‐95169 from the National Heart, Lung, and Blood Institute and by grants UL1‐TR‐000040 and UL1‐TR‐001079 from NCRR. Dr Bertoni was supported with NIH/NHLBI R01 grant, R01HL127028.
Disclosures
Dr Kestenbaum received consulting fees from Sanofi Inc. The remaining authors have no disclosures to report.
Supporting information
Table S1. Associations of FGF‐23 and Incident Unclassified HF (Hazard Ratios for Each 20 pg/mL Higher FGF‐23 Concentration): The Multi‐Ethnic Study of Atherosclerosis
Table S2. Associations of FGF‐23 and Incident HFpEF Before and After Adjustment for LV Mass (Hazard Ratios for Each 20 pg/mL Higher FGF‐23 Concentration): The Multi‐Ethnic Study of Atherosclerosis
Table S3. Associations of FGF‐23 and Incident HF, HFrEF and HFpEF (Hazard Ratios for Each 20 pg/mL Higher FGF‐23 Concentration Using Time‐Updated Covariates): The Multi‐Ethnic Study of Atherosclerosis
Table S4. Associations of FGF‐23 and Incident HFrEF and HFpEF (Hazard Ratios for Each 20 pg/mL Higher FGF‐23 Concentration Using Complete Case Analysis): The Multi‐Ethnic Study of Atherosclerosis
Acknowledgments
The authors thank the investigators, staff, and participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
(J Am Heart Assoc. 2018;7:e008334 DOI: 10.1161/JAHA.117.008334.)
References
- 1. Schocken DD, Benjamin EJ, Fonarow GC, Krumholz HM, Levy D, Mensah GA, Narula J, Shor ES, Young JB, Hong Y. Prevention of heart failure: a scientific statement from the American Heart Association Councils on Epidemiology and Prevention, Clinical Cardiology, Cardiovascular Nursing, and High Blood Pressure Research; Quality of Care and Outcomes Research Interdisciplinary Working Group; and Functional Genomics and Translational Biology Interdisciplinary Working Group. Circulation. 2008;117:2544–2565. [DOI] [PubMed] [Google Scholar]
- 2. Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Das SR, de Ferranti S, Despres JP, Fullerton HJ, Howard VJ, Huffman MD, Isasi CR, Jimenez MC, Judd SE, Kissela BM, Litchman JH, Lisabeth LD, Liu S, Mackey RH, Magid DJ, McGuire DK, Mohler ER 3rd, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Rosamond W, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Woo D, Yeh RW, Turner MB. Heart disease and stroke statistics‐2016 update: a report from the American Heart Association. Circulation. 2016;133:e38–e60. [DOI] [PubMed] [Google Scholar]
- 3. McMurray JJ, Petrie MC, Murdoch DR, Davie AP. Clinical epidemiology of heart failure: public and private health burden. Eur Heart J. 1998;19 (suppl P):P9–P16. [PubMed] [Google Scholar]
- 4. Steinberg BA, Zhao X, Heidenreich PA, Peterson ED, Bhatt DL, Cannon CP, Hernandez AF, Fonarow GC. Trends in patients hospitalized with heart failure and preserved left ventricular ejection fraction: prevalence, therapies, and outcomes. Circulation. 2012;126:65–75. [DOI] [PubMed] [Google Scholar]
- 5. Loh JC, Creaser J, Rourke DA, Livingston N, Harrison TK, Vandenbogaart E, Moriguchi J, Hamilton MA, Tseng CH, Fonarow GC, Horwich TB. Temporal trends in treatment and outcomes for advanced heart failure with reduced ejection fraction from 1993–2010: findings from a university referral center. Circ Heart Fail. 2013;6:411–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chan MM, Lam CS. How do patients with heart failure with preserved ejection fraction die? Eur J Heart Fail. 2013;15:604–613. [DOI] [PubMed] [Google Scholar]
- 7. Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Secular trends in renal dysfunction and outcomes in hospitalized heart failure patients. J Cardiac Fail. 2006;12:257–262. [DOI] [PubMed] [Google Scholar]
- 8. Yancy CW, Lopatin M, Stevenson LW, De Marco T, Fonarow GC, Committee ASA. Clinical presentation, management, and in‐hospital outcomes of patients admitted with acute decompensated heart failure with preserved systolic function: a report from the acute Decompensated Heart Failure National Registry (adhere) database. J Am Coll Cardiol. 2006;47:76–84. [DOI] [PubMed] [Google Scholar]
- 9. McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Bohm M, Dickstein K, Falk V, Filippatos G, Fonseca C, Gomez‐Sanchez MA, Jaarsma T, Kober L, Lip GY, Maggioni AP, Parkhomenko A, Pieske BM, Popescu BA, Ronnevik PK, Rutten FH, Schwitter J, Seferovic P, Stepinska J, Trindade PT, Voors AA, Zannad F, Zeiher A. Esc guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: the task force for the diagnosis and treatment of acute and chronic heart failure 2012 of the European Society of Cardiology. developed in collaboration with the heart failure association (HFA) of the esc. Eur J Heart Fail. 2012;14:803–869. [DOI] [PubMed] [Google Scholar]
- 10. Shah AM, Pfeffer MA. The many faces of heart failure with preserved ejection fraction. Nat Rev Cardiol. 2012;9:555–556. [DOI] [PubMed] [Google Scholar]
- 11. Oktay AA, Rich JD, Shah SJ. The emerging epidemic of heart failure with preserved ejection fraction. Curr Heart Fail Rep. 2013;10:401–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bhuiyan T, Maurer MS. Heart failure with preserved ejection fraction: persistent diagnosis, therapeutic enigma. Curr Cardiovas Risk Rep. 2011;5:440–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cleland JG, Pellicori P. Defining diastolic heart failure and identifying effective therapies. JAMA. 2013;309:825–826. [DOI] [PubMed] [Google Scholar]
- 14. Larsson T, Marsell R, Schipani E, Ohlsson C, Ljunggren O, Tenenhouse HS, Juppner H, Jonsson KB. Transgenic mice expressing fibroblast growth factor 23 under the control of the alpha1(I) collagen promoter exhibit growth retardation, osteomalacia, and disturbed phosphate homeostasis. Endocrinology. 2004;145:3087–3094. [DOI] [PubMed] [Google Scholar]
- 15. Gattineni J, Baum M. Regulation of phosphate transport by fibroblast growth factor 23 (FGF23): implications for disorders of phosphate metabolism. Pediatr Nephrol. 2010;25:591–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Shimada T, Hasegawa H, Yamazaki Y, Muto T, Hino R, Takeuchi Y, Fujita T, Nakahara K, Fukumoto S, Yamashita T. FGF‐23 is a potent regulator of vitamin d metabolism and phosphate homeostasis. J Bone Miner Res. 2004;19:429–435. [DOI] [PubMed] [Google Scholar]
- 17. Ix JH, Katz R, Kestenbaum BR, de Boer IH, Chonchol M, Mukamal KJ, Rifkin D, Siscovick DS, Sarnak MJ, Shlipak MG. Fibroblast growth factor‐23 and death, heart failure, and cardiovascular events in community‐living individuals: CHS (cardiovascular health study). J Am Coll Cardiol. 2012;60:200–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Faul C, Amaral AP, Oskouei B, Hu MC, Sloan A, Isakova T, Gutierrez OM, Aguillon‐Prada R, Lincoln J, Hare JM, Mundel P, Moralez A, Scialla J, Fischer M, Soliman EZ, Chen J, Go AS, Rosas SE, Nessel L, Townsend RR, Feldman HI, St John Sutton M, Ojo A, Gadegbeku C, Di Marco GS, Reuter S, Kentrup D, Tiemann K, Brand M, Hill JA, Moe OW, Juro OM, Kusek JW, Keane MG, Wolf M. FGF23 induces left ventricular hypertrophy. J Clin Investig. 2011;121:4393–4408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kestenbaum B, Sachs MC, Hoofnagle AN, Siscovick DS, Ix JH, Robinson‐Cohen C, Lima JA, Polak JF, Blondon M, Ruzinski J, Rock D, De Boer IH. Fibroblast growth factor‐23 and cardiovascular disease in the general population: the multi‐ethnic study of atherosclerosis. Circ Heart Fail. 2014;7:409–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Desjardins L, Liabeuf S, Renard C, Lenglet A, Lemke HD, Choukroun G, Drueke TB, Massy ZA; European Uremic Toxin Work Group . FGF23 is independently associated with vascular calcification but not bone mineral density in patients at various CKD stages. Osteoporos Int. 2012;23:2017–2025. [DOI] [PubMed] [Google Scholar]
- 21. Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, Greenland P, Jacob DR Jr, Kronmal R, Liu K, Nelson JC, O'Leary D, Saad MF, Shea S, Szklo M, Tracy RP. Multi‐ethnic study of atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–881. [DOI] [PubMed] [Google Scholar]
- 22. Inker LA, Schmid CH, Tighiouart H, Eckfeldt JH, Feldman HI, Greene T, Kusek JW, Manzi J, Van Lente F, Zhang YL, Coresh J, Levey AS; CKD‐EPI Investigators . Estimating glomerular filtration rate from serum creatinine and cystatin c. N Engl J Med. 2012;367:20–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Choi EY, Bahrami H, Wu CO, Greenland P, Cushman M, Daniels LB, Almeida AL, Yoneyama K, Opdahl A, Jain A, Criqui MH, Siscovick D, Darwin C, Maisel A, Bluemke DA, Lima JA. N‐terminal pro‐B‐type natriuretic peptide, left ventricular mass, and incident heart failure: multi‐ethnic study of atherosclerosis. Circ Heart Fail. 2012;5:727–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Imel EA, Peacock M, Pitukcheewanont P, Heller HJ, Ward LM, Shulman D, Kassem M, Rackoff P, Zimering M, Dalkin A, Drobny E, Colussi G, Shaker JL, Hoogendoorn EH, Hui SL, Econs MJ. Sensitivity of fibroblast growth factor 23 measurements in tumor‐induced osteomalacia. J Clin Endocrinol Metab. 2006;91:2055–2061. [DOI] [PubMed] [Google Scholar]
- 25. Royston P. Multiple imputation of missing values. Stata J. 2004;4:227–241. [Google Scholar]
- 26. Rubin DB. Multiple Imputation for Nonresponse in Surveys. Hoboken: Wiley Publishing; 1987. [Google Scholar]
- 27. Mirza MA, Larsson A, Melhus H, Lind L, Larsson TE. Serum intact FGF23 associate with left ventricular mass, hypertrophy and geometry in an elderly population. Atherosclerosis. 2009;207:546–551. [DOI] [PubMed] [Google Scholar]
- 28. Fyfe‐Johnson AL, Alonso A, Selvin E, Bower JK, Pankow JS, Agarwal SK, Lutsey PL. Serum fibroblast growth factor‐23 and incident hypertension: the atherosclerosis risk in communities (ARIC) study. J Hypertens. 2016;34:1266–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Grabner A, Schramm K, Silswal N, Hendrix M, Yanucil C, Czaya B, Singh S, Wolf M, Hermann S, Stypmann J, Di Marco GS, Brand M, Wacker MJ, Faul C. FGF23/FGFR4‐mediated left ventricular hypertrophy is reversible. Sci Rep. 2017;7:1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Dai B, David V, Martin A, Huang J, Li H, Jiao Y, Gu W, Quarlez LD. A comparative transcriptome analysis identifying FGF23 regulated genes in the kidney of a mouse CKD model. PLoS One. 2012;7:e44161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gutierrez O, Isakova T, Rhee E, Shah A, Holmes J, Collerone G, Juppner H, WolF M. Fibroblast growth factor‐23 mitigates hyperphosphatemia but accentuates calcitriol deficiency in chronic kidney disease. J Am Soc Nephrol. 2005;16:2205–2215. [DOI] [PubMed] [Google Scholar]
- 32. Munoz Mendoza J, Isakova T, Ricardo AC, Xie H, Navaneethan SD, Anderson AH, Bazzano LA, Xie D, Kretzler M, Nessel L, Hamm LL, Negrea L, Leonard MB, Raj D, Wolf M; Chronic Renal Insufficiency Cohort . Fibroblast growth factor 23 and inflammation in CKD. Clin J Am Soc Nephrol. 2012;7:1155–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Andrukhova O, Slavic S, Smorodchenko A, Zeitz U, Shalhoub V, Lanske B, Pohl EE, Erben RG. FGF23 regulates renal sodium handling and blood pressure. EMBO Mol Med. 2014;6:744–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Isakova T, Wahl P, Vargas GS, Gutierrez OM, Scialla J, Xie H, Appleby D, Nessel L, Bellovich K, Chen J, Hamm L, Gadegbeku C, Horwitz E, Townsend RR, Anderson CA, Lash JP, Hsu CY, Leonard MB, Wolf M. Fibroblast growth factor 23 is elevated before parathyroid hormone and phosphate in chronic kidney disease. Kidney Int. 2011;79:1370–1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hu MC, Shi M, Cho HJ, Adams‐Huet B, Paek J, Hill K, Shelton J, Amaral AP, Faul C, Taniguchi M, Wolf M, Brand M, Takahashi M, Kuro OM, Hill JA, Moe OW. Klotho and phosphate are modulators of pathologic uremic cardiac remodeling. J Am Soc Nephrol. 2015;26:1290–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Xie J, Cha SK, An SW, Kuro OM, Birnbaumer L, Huang CL. Cardioprotection by Klotho through downregulation of TRPC6 channels in the mouse heart. Nat Commun. 2012;3:1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Reindl M, Reinstadler SJ, Feistritzer HJ, Mueller L, Koch C, Mayr A, Theurl M, Kirchmair R, Klug G, Metzler B. Fibroblast growth factor 23 as novel biomarker for early risk stratification after ST‐elevation myocardial infarction. Heart. 2017;103:856–862. [DOI] [PubMed] [Google Scholar]
- 38. Hao H, Li X, Li Q, Lin H, Chen Z, Xie J, Xuan W, Liao W, Bin J, Huang X, Kitakaze M, Liao Y. FGF23 promotes myocardial fibrosis in mice through activation of beta‐catenin. Oncotarget. 2016;7:64649–64664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Velagaleti RS, Gona P, Pencina MJ, Aragam J, Wang TJ, Levy D, D'Agostino RB, Lee DS, Kannel WB, Benjamin EJ, Vasan RS. Left ventricular hypertrophy patterns and incidence of heart failure with preserved versus reduced ejection fraction. Am J Cardiol. 2014;113:117–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bluemke DA, Kronmal RA, Lima JA, Liu K, Olson J, Burke GL, Folsom AR. The relationship of left ventricular mass and geometry to incident cardiovascular events: the MESA (Multi‐Ethnic Study of Atherosclerosis) Study. J Am Coll Cardiol. 2008;52:2148–2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Seliger SL, de Lemos J, Neeland IJ, Christenson R, Gottdiener J, Drazner MH, Berry J, Sorkin J, deFilippi C. Older adults, “malignant” left ventricular hypertrophy, and associated cardiac‐specific biomarker phenotypes to identify the differential risk of new‐onset reduced versus preserved ejection fraction heart failure: CHS (cardiovascular health study). JACC Heart Fail. 2015;3:445–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Agarwal I, Ide N, Ix JH, Kestenbaum B, Lanske B, Schiller NB, Whooley MA, Mukamal KJ. Fibroblast growth factor‐23 and cardiac structure and function. J Am Heart Assoc. 2014;3:e000584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Tanaka S, Fujita S, Kizawa S, Morita H, Ishizaka N. Association between FGF23, alpha‐Klotho, and cardiac abnormalities among patients with various chronic kidney disease stages. PLoS One. 2016;11:e0156860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Seiler S, Cremers B, Rebling NM, Hornof F, Jeken J, Kersting S, Steimle C, Ege P, Fehrenz M, Rogacev KS, Scheller B, Bohm M, Fliser D, Heine GH. The phosphatonin fibroblast growth factor 23 links calcium‐phosphate metabolism with left‐ventricular dysfunction and atrial fibrillation. Eur Heart J. 2011;32:2688–2696. [DOI] [PubMed] [Google Scholar]
- 45. Bienaime F, Dechartres A, Anglicheau D, Sabbah L, Montgermont P, Friedlander G, Ravaud P, Legendre C, Prie D. The association between fibroblast growth factor 23 and renal transplantation outcome is modified by follow‐up duration and glomerular filtration rate assessment method. Kidney Int Rep. 2017;2:881–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ndumele CE, Matsushita K, Sang Y, Lazo M, Agarwal SK, Nambi V, Deswal A, Blumenthal RS, Ballantyne CM, Coresh J, Selvin E. N‐terminal pro‐brain natriuretic peptide and heart failure risk among individuals with and without obesity: the atherosclerosis risk in communities (ARIC) study. Circulation. 2016;133:631–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Patton KK, Heckbert SR, Alonso A, Bahrami H, Lima JA, Burke G, Kronmal RA. N‐terminal pro‐B‐type natriuretic peptide as a predictor of incident atrial fibrillation in the multi‐ethnic study of atherosclerosis: the effects of age, sex and ethnicity. Heart. 2013;99:1832–1836. [DOI] [PubMed] [Google Scholar]
- 48. Sanchez OA, Duprez DA, Bahrami H, Daniels LB, Folsom AR, Lima JA, Maisel A, Peralta CA, Jacobs DR Jr. The associations between metabolic variables and NT‐proBNP are blunted at pathological ranges: the Multi‐Ethnic Study of Atherosclerosis. Metabolism. 2014;63:475–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Scialla JJ, Xie H, Rahman M, Anderson AH, Isakova T, Ojo A, Zhang X, Nessel L, Hamano T, Grunwald JE, Raj DS, Yang W, He J, Lash JP, Go AS, Kusek JW, Feldman H, Wolf M; Chronic Renal Insufficiency Cohort Study Investigators . Fibroblast growth factor‐23 and cardiovascular events in CKD. J Am Soc Nephrol. 2014;25:349–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Associations of FGF‐23 and Incident Unclassified HF (Hazard Ratios for Each 20 pg/mL Higher FGF‐23 Concentration): The Multi‐Ethnic Study of Atherosclerosis
Table S2. Associations of FGF‐23 and Incident HFpEF Before and After Adjustment for LV Mass (Hazard Ratios for Each 20 pg/mL Higher FGF‐23 Concentration): The Multi‐Ethnic Study of Atherosclerosis
Table S3. Associations of FGF‐23 and Incident HF, HFrEF and HFpEF (Hazard Ratios for Each 20 pg/mL Higher FGF‐23 Concentration Using Time‐Updated Covariates): The Multi‐Ethnic Study of Atherosclerosis
Table S4. Associations of FGF‐23 and Incident HFrEF and HFpEF (Hazard Ratios for Each 20 pg/mL Higher FGF‐23 Concentration Using Complete Case Analysis): The Multi‐Ethnic Study of Atherosclerosis


