Abstract
Background:
Prospectively collected frailty markers are associated with an incremental 1-year mortality risk after transcatheter aortic valve replacement (TAVR) compared to comorbidities alone. Whether information on frailty markers captured retrospectively in administrative billing data is similarly predictive of long-term mortality after TAVR is unknown. We sought to characterize the prognostic importance of frailty factors as identified in healthcare billing records in comparison to validated measures of frailty for the prediction of long-term mortality after TAVR.
Methods and Results:
Adult patients undergoing TAVR between August 25, 2011 and September 29, 2015 were identified among Medicare fee-for-service beneficiaries. The Johns Hopkins Claims-based Frailty Indicator was used to identify frail patients. We used nested Cox regression models to identify claims-based predictors of mortality up to 4 years post-procedure. Four groups of variables including cardiac risk factors, non-cardiac risk factors, patient procedural risk factors, and non-traditional markers of frailty were introduced sequentially, and their integrated discrimination improvement (IDI) was assessed. A total of 52,338 TAVR patients from 558 clinical sites were identified, with a mean follow-up time period of 16 months. In total, 14,174 (27.1%) patients died within the study period. The mortality rate was 53.9% at 4-years post TAVR. A total of 34,863 (66.6%) patients were defined as frail. The discrimination of each of the 4 models was 0.60 (95% CI: 0.59–60), 0.65 (95% CI: 0.64–0.65), 0.68 (95% CI: 0.67–0.68) and 0.70 (95% CI: 0.69–0.70), respectively. The addition of non-traditional frailty markers as identified in claims improved mortality prediction above and beyond traditional risk factors (IDI: 0.019, p < 0.001).
Conclusions:
Risk prediction models that include frailty as identified in claims data can be used to predict long-term mortality risk after TAVR. Linkage to claims data may allow enhanced mortality risk prediction for studies that do not collect information on frailty.
INTRODUCTION
Severe calcific aortic valve stenosis is the most common cause of valve replacement in the elderly population in the United States (US).1 While surgical aortic valve replacement (SAVR) remains the preferred treatment for severe symptomatic aortic stenosis in patients at low surgical risk, current guidelines recommend transcatheter aortic valve replacement (TAVR) as an alternative treatment in patients at increased surgical risk based on a number of clinical trials showing equivalence or superiority to surgery for patients with extreme, high, and intermediate surgical risk 2–8.
Risk stratification before TAVR is important when selecting those patients who will most likely benefit from the procedure. To date, clinical risk prediction models of 1-year mortality after TAVR have been developed using traditional risk scoring systems. These include the Society for Thoracic Surgery Predicted Risk of Mortality (STS-PROM) score 9, 10 and the logistic EuroSCORE 11, as well as other individual clinical risk predictors 12–16. Prior studies have suggested that measurement of frailty can enhance the prediction of mortality after TAVR 17–30. Additionally, a relatively small, prospective study recently showed that adding frailty measurements to conventional risk scores improved the assessment of 1-year mortality risk after TAVR 31.
However, the assessment of frailty in clinical practice can be challenging, and these factors are commonly not collected in the nationwide registries such as the Society of Thoracic Surgeons (STS)/American College of Cardiology (ACC) Transcatheter Valve Therapy (TVT) Registry. In the absence of prospectively collected data, the ability to identify these factors in billing records may allow for enhanced mortality prediction. To test this, we evaluated hospitalizations of patients undergoing TAVR in a Medicare inpatient database to determine whether the incorporation of claims-based measures of frailty might augment mortality prediction compared to using comorbidities alone.
METHODS
The data, analytic methods, and study materials will not be made available to other researchers for purposes of reproducing the results or replicating the procedure.
Study Population
The Centers for Medicare and Medicaid Services (CMS) MedPAR files include administrative billing claims for all hospitalizations of Medicare fee-for-service beneficiaries, and have been used to study national patterns of procedure utilization in the US 32–34. In the MedPAR database, hospitalizations of adult patients (≥18 years old) were included if they had at least one International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) code for either transfemoral (35.05) or transapical (35.06) TAVR performed between August 25, 2011 and September 29, 2015.
Cardiac and Non-cardiac Covariates and Procedural Risk Factors
A total of 34 cardiac and non-cardiac covariates and procedural risk factors were initially defined as possible risk factors for all-cause mortality after TAVR based on our clinical knowledge. Age, gender, race, chronic heart failure, smoking, peripheral vascular disease, diabetes mellitus and a couple of well-known cardiac diseases were defined as cardiac risk factors. Other comorbidities such chronic kidney, liver and obstructive pulmonary diseases, anemia, obesity, hypothyroidism, coagulopathy and the Charlson comorbidity index were defined as non-cardiac risk factors. Procedural risk factors included emergency or urgent admission, transapical access and pre-procedural shock.
Frailty Index
There are several ways to measure claims-based frailty 35–36. In this study, the Johns Hopkins Claims-based Frailty Indicator, a previously developed and both internally and externally validated index, was used to identify frailty 37–40. This index includes 21 criteria identifiable in claims data, such as demographic variables and markers of physical and cognitive dysfunction, to identify patients meeting Fried’s Frailty Phenotype 41. This has been extensively validated and shown to predict poor health outcomes including incidence of falls, worsening mobility, hospitalization, and death. Based on the Johns Hopkins Claims-based Frailty Indicator algorithm 37, a score cutoff of ≥ 0.12 was used to identify frail patients (Supplementary Table 1).
Some variables included in the John Hopkins Claims-based Frailty Indicator, such as age, sex, and Charlson comorbidity index, overlap with variables commonly used for risk adjustment in TAVR patients. In order to examine the prognostic importance of frailty variables often omitted from risk adjustment, we classified a subgroup of variables in the Frailty Indicator as non-traditional frailty markers. These included impaired mobility, depression, Parkinson’s disease, any type of arthritis, cognitive impairment, paranoia, chronic skin ulcer, pneumonia, skin and soft tissue infections, mycoses, gout or other crystal-induced arthropathies, falls, musculosketal problems and urinary tract infection.
All covariates were ascertained using secondary diagnosis codes that were coded as “present on admission” during the index hospitalization, as well as from principal and secondary diagnosis codes from all hospitalizations in the year prior to the date of admission of the index hospitalization (Supplementary Table 2).
Outcomes
The primary outcome for this study was all-cause long-term mortality, determined through linkage of the MedPAR files to the CMS denominator file which includes information on a patient’s vital status. Time to death was calculated as the time between the date of procedure and date of death. Patients were censored if they were no longer enrolled in Medicare according to the denominator file as of December 31, 2015, which marked the end of the follow-up period. The study was approved by the institutional review board of Beth Israel Deaconess Medical Center with a waiver of informed consent for retrospective data analysis.
Statistical Analysis
Continuous variables are presented as means and standard deviations, and categorical variables are presented as counts and percentages. Covariates were compared between surviving and non-surviving patients using chi-square statistics and t-tests. Kaplan-Meier plots were created to plot time to death with a 30-day landmark analysis, stratified by the number of non-traditional frailty markers included. The log-rank test was used to compare the survival distributions of each frailty scale.
Covariates with a p-value of <0.1 in univariate Cox regression analysis were ultimately included in the model. Subsequently, nested multivariable Cox regression incorporating random hospital effects was performed using four sequential models to determine the incremental improvement in prediction of long-term mortality with the addition of four sets of covariates. The sequential models included variables associated with 1) cardiac risk factors, 2) non-cardiac risk factors, 3) procedural risk factors and 4) non-traditional frailty markers. Harrell’s c-statistic was used to assess model discrimination, and the improvement in discrimination with the addition of variables was assessed by the change in the c-statistic and the DeLong test 42. An integrated discrimination improvement (IDI) test was used to assess discrimination improvement 43. All statistical analyses were performed in STATA software, version 15.0 (Stata Corporation, College Station, TX) using a two-tailed p-value for significance of <0.05.
RESULTS
A total of 52,338 hospitalizations from 558 clinical sites involved receipt of TAVR over the study period. The baseline characteristics of patients are shown in Table 1. A total of 27,147 (51.9%) were men and the mean age was 82.4 ±8.0 years. There were 34,863 (66.6%) patients that were identified as frail. Musculoskeletal problems (15.5%) and arthritis (13.9%) were the most prevalent non-traditional frailty markers.
Table 1.
Characteristics of the study population between surviving and dead patients
| Overall n=52,338 | Alive n=38,164 (72.9%) | Dead n=14,174 (27.1%) | p-value | |
|---|---|---|---|---|
| Cardiac History | ||||
| Age, years (mean±SD) | 82.4±8.0 | 82.2±7.8 | 82.9±8.3 | <0.001 |
| Men, no. of pts (%) | 27,147 (51.9) | 19,444 (50.1) | 7,703 (54.3) | <0.001 |
| White race, no. of pts (%) | 48,635 (92.9) | 35,414 (92.8) | 13,221 (93.3) | 0.067 |
| Chronic heart failure, no. of pts (%) | 39,416 (75.3) | 27,982 (73.3) | 11,434 (80.7) | <0.001 |
| Diabetes mellitus, no. of pts (%) | 19,062 (36.4) | 13,915 (36.5) | 5,147 (36.3) | 0.15 |
| Smoker, no. of pts (%) | 16,679 (31.9) | 12,667 (33.2) | 4,012 (28.3) | <0.001 |
| Coronary artery disease without revascularization, no. of pts (%) | 37,770 (72.2) | 27,783 (72.8) | 9,987 (70.5) | <0.001 |
| Prior myocardial infarction, no. of pts (%) | 8,419 (16.1) | 6,057 (15.9) | 2,362 (16.7) | 0.028 |
| Prior percutaneous coronary intervention, no. of pts (%) | 11,465 (21.9) | 8,693 (22.8) | 2,772 (19.6) | <0.001 |
| Prior valvular surgery, no. of pts (%) | 855 (1.6) | 649 (1.7) | 206 (1.5) | 0.047 |
| Prior aortic surgery, no. of pts (%) | 211 (0.4) | 140 (0.4) | 71 (0.5) | 0.031 |
| Prior coronary artery bypass graft surgery, no. of pts (%) | 12,073 (23.1) | 9,133 (23.9) | 2,940 (20.7) | <0.001 |
| Peripheral vascular disease, no. of pts (%) | 5,382 (10.3) | 3,853 (10.1) | 1,529 (10.8) | 0.021 |
| Atrial fibrillation, no. of pts (%) | 24,567 (46.9) | 16,671 (43.7) | 7,896 (55.7) | <0.001 |
| Center bundle branch block, no. of pts (%) | 5,502 (10.5) | 4,323 (11.3) | 1,179 (8.3) | <0.001 |
| Right bundle branch block, no. of pts (%) | 2,000 (3.8) | 1,555 (4.1) | 445 (3.1) | <0.001 |
| Cerebrovascular disease, no. of pts (%) | 7,230 (13.8) | 5,108 (13.4) | 2,122 (15.0) | <0.001 |
| Tricuspid valve disorders, no. of pts (%) | 4,557 (8.7) | 3,177 (8.3%) | 1,380 (9.7%) | <0.001 |
| Endocarditis, no. of pts (%) | 45 (0.1) | 23 (0.1) | 22 (0.2) | 0.001 |
| Aortic aneurysm, no. of pts (%) | 1,876 (3.6) | 1,283 (3.4) | 593 (4.2) | 0.001 |
| Non-Cardiac History | ||||
| Chronic kidney disease without dialysis, no. of pts (%) | 21,071 (40.3) | 14,113 (37.0) | 6,958 (49.1) | <0.001 |
| Renal dialysis, no. of pts (%) | 1,269 (2.4) | 714 (1.9) | 555 (3.9) | <0.001 |
| Liver disease, no. of pts (%) | 1,915 (3.7) | 1,042 (2.7) | 873 (6.2) | <0.001 |
| Chronic obstructive pulmonary disease, no. of pts (%) | 17,566 (33.6) | 12,226 (32.0) | 5,340 (37.7) | <0.001 |
| Home O2, no. of pts (%) | 3,551 (6.8) | 2,207 (5.8) | 1,344 (9.5) | <0.001 |
| Hypothyroidism, no. of pts (%) | 11,456 (21.9) | 8,311 (21.8) | 3,145 (22.2) | 0.31 |
| Coagulopathy, no. of pts (%) | 10,285 (19.7) | 6,973 (18.3) | 3,312 (23.4) | <0.001 |
| Obesity, no. of pts (%) | 7,852 (15.0) | 6,197 (16.2) | 1,655 (11.7) | <0.001 |
| Anemia, no. of pts (%) | 26,328 (50.3) | 18,282 (47.9) | 8,046 (56.8) | <0.001 |
| Charlson Comorbidity Index, (mean±SD) | 3.30±1.87 | 3.14±1.83 | 3.74±1.92 | <0.001 |
| Procedural Characteristics | ||||
| Emergency admission, no. of pts (%) | 3,819 (7.3) | 2,396 (6.3) | 1,423 (10.0) | <0.001 |
| Urgent admission, no. of pts (%) | 7,377 (14.1) | 4,927 (12.9) | 2,450 (17.3) | <0.001 |
| Transapical, no. of pts (%) | 8,071 (15.4) | 5,218 (13.7) | 2,853 (20.1) | <0.001 |
| Pre-procedural shock, no. of pts (%) | 1,423 (2.7) | 535 (1.4) | 888 (6.3) | <0.001 |
| Non-Traditional Frailty Markers | ||||
| Impaired mobility, no. of pts (%) | 314 (0.6) | 212 (0.6) | 102 (0.7) | 0.031 |
| Depression, no. of pts (%) | 4,243 (8.1) | 3,099 (8.1) | 1,144 (8.1) | 0.85 |
| Parkinson’s Disease, no. of pts (%) | 717 (1.4) | 517 (1.4) | 200 (1.4) | 0.62 |
| Arthritis (Any Type), no. of pts (%) | 7,290 (13.9) | 5,537 (14.5) | 1,753 (12.4) | <0.001 |
| Cognitive Impairment, no. of pts (%) | 4,924 (9.4) | 3,381 (8.9) | 1,543 (10.9) | <0.001 |
| Paranoia, no. of pts (%) | 493 (0.9) | 326 (0.9) | 167 (1.2) | <0.001 |
| Chronic Skin Ulcer, no. of pts (%) | 1,283 (2.5) | 629 (1.6) | 654 (4.6) | <0.001 |
| Pneumonia, no. of pts (%) | 1,782 (3.5) | 777 (2.0) | 1,005 (7.3) | <0.001 |
| Falls, no. of pts (%) | 60 (0.1) | 36 (0.1) | 24 (0.1) | 0.20 |
| Skin and Soft Tissue Infections, no. of pts (%) | 515 (1.0) | 304 (0.8) | 211 (1.5) | <0.001 |
| Mycoses, no. of pts (%) | 748 (1.4) | 379 (1.0) | 369 (2.6) | <0.001 |
| Gout or Other Crystal-Induced Arthropathies, no. of pts (%) | 3,309 (6.3) | 2,387 (6.3) | 922 (6.5) | 0.30 |
| Musculosketal Problems, no. of pts (%) | 8,101 (15.5) | 6,244 (16.4) | 1,857 (13.1) | <0.001 |
| Urinary Tract Infection, no. of pts (%) | 4,888 (9.3) | 3,032 (7.9) | 1,856 (13.1) | <0.001 |
SD = standard deviation
Mortality
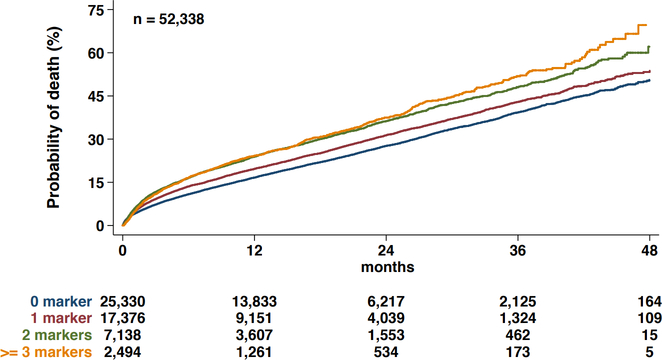
In total, 14,174 (27.1%) patients died within the study period, with a mean follow-up time of 16 months. The all-cause mortality rate was 3.6% in-hospital, 5.1% at 30-days, 19.0% at 1-year, 30.1% at 2-years, 42.3% at 3-years, and 53.9% at 4-years post-TAVR. Kaplan–Meier curves showing overall mortality up to 4 years after TAVR stratified by the number of non-traditional frailty markers are shown in Figure 1. Compared to those without any non-traditional frailty markers, the hazard ratio (HR) was 1.166 (95% confidence interval [CI]: 1.122–1.211) for patients with one frailty marker, 1.417 (95% CI: 1.350–1.488) for those with two frailty markers, and 1.490 (95% CI: 1.383–1.610) for those with ≥3 frailty markers (p<0.001 for all). The predicted 4-year mortality was 57% for frail patients and 48% for non-frail patients (HR = 1.342, 95% CI: 1.293–1.393, p<0.001). In the 30-day landmark analysis, compared to those without any non-traditional frailty markers, the HR was 1.010 (95% CI: 0.925–1.144) for patients with one frailty marker 1.185 (95% CI: 0.955–1.308) for those with two frailty markers, and 1.136 (95% CI: 0.935–1.380) for those with ≥3 frailty markers (p=0.135).
Figure 1.
Kaplan Meier curves for all-cause mortality according to presence of non-traditional frailty markers
Discrimination Improvement and Model Covariates
In nested models, the discrimination of the first model including only cardiac risk factors was 0.60 (95% CI: 0.59–60). The addition of non-cardiac risk factors in the second model resulted in a c-statistic of 0.65 (95% CI: 0.64–0.65) (IDI=0.032, p<0.001 compared to the first model). With the incorporation of additional procedural characteristics in the third model, the c-statistic was 0.68 (95% CI: 0.67–0.68) (IDI=0.019, p<0.001 compared to the second model). Finally, the inclusion of non-traditional frailty markers resulted in a c-statistic of 0.70 (95% CI: 0.69–0.70) (IDI=0.019, p<0.001 compared to third model). Comparisons of the c-statistics using the DeLong test were statistically significant (p<0.001) for each model (Model 2 vs Model 1, Model 3 vs Model 2, Model 4 vs Model 3) (Table 2).
Table 2.
Results of multivariable nested Cox regression
| Hazard Ratio | 95% CIs | p-value | |
|---|---|---|---|
| Cardiac History | |||
| Age (by year) | 1.015 | 1.013–1.018 | <0.001 |
| Male | 1.223 | 1.179–1.269 | <0.001 |
| Chronic heart failure | 1.249 | 1.195–1.304 | <0.001 |
| Diabetes mellitus | 1.047 | 1.009–1.086 | 0.013 |
| Smoker | 0.886 | 0.852–0.921 | <0.001 |
| Coronary artery disease without revascularization | 0.924 | 0.887–0.962 | <0.001 |
| Prior percutaneous coronary intervention | 0.930 | 0.889–0.972 | 0.001 |
| Prior coronary artery bypass graft surgery | 0.883 | 0.845–0.923 | <0.001 |
| Atrial fibrillation | 1.394 | 1.347–1.442 | <0.001 |
| Center bundle branch block | 0.893 | 0.841–0.948 | <0.001 |
| Right bundle branch block | 0.840 | 0.763–0.924 | 0.004 |
| Aortic aneurysm | 1.141 | 1.046–1.244 | 0.003 |
| Non-Cardiac History | |||
| Chronic kidney disease without dialysis | 1.378 | 1.330–1.427 | <0.001 |
| Renal dialysis | 2.033 | 1.844–2.411 | <0.001 |
| Liver disease | 2.046 | 1.905–2.197 | <0.001 |
| Chronic obstructive pulmonary disease | 1.198 | 1.155–1.243 | <0.001 |
| Home O2 | 1.556 | 1.466–1.652 | <0.001 |
| Coagulopathy | 1.100 | 1.057–1.145 | <0.001 |
| Obesity | 0.789 | 0.748–0.833 | <0.001 |
| Anemia | 1.132 | 1.093–1.172 | <0.001 |
| Charlson Comorbidity Index | 1.136 | 1.125–1.146 | <0.001 |
| Procedural Characteristics | |||
| Emergency admission | 1.298 | 1.225–1.374 | <0.001 |
| Urgent admission | 1.166 | 1.114–1.220 | <0.001 |
| Transapical | 1.123 | 1.076–1.172 | 0.020 |
| Pre-procedural shock | 2.369 | 2.201–2.549 | <0.001 |
| non-Traditional Frailty Markers | |||
| Impaired mobility | 1.240 | 1.015–1.516 | 0.035 |
| Depression | 1.067 | 1.003–1.135 | 0.038 |
| Cognitive Impairment | 1.229 | 1.165–1.297 | 0.001 |
| Paranoia | 1.252 | 1.075–1.459 | 0.004 |
| Chronic Skin Ulcer | 1.670 | 1.540–1.811 | <0.001 |
| Pneumonia | 1.883 | 1.761–2.014 | <0.001 |
| Skin and Soft Tissue Infections | 1.269 | 1.106–1.457 | <0.001 |
| Mycoses | 1.446 | 1.301–1.607 | <0.001 |
| Urinary Tract Infection | 1.279 | 1.215–1.345 | <0.001 |
CI = confidence interval
The hazard ratios for the final covariates, determined from nested cox regression analysis, are presented in Table 3. The covariates that were most strongly associated with increased long-term mortality were atrial fibrillation (HR: 1.394, 95% CI: 1.347–1.442, p<0.001), chronic heart failure (HR: 1.249, 95% CI: 1.195–1.304, p<0.001) , dialysis (HR: 2.033, 95% CI: 1.844–2.411, p<0.001), liver disease (HR: 2.046, 95% CI: 1.905–2.197, p<0.001), emergency admission (HR: 1.298, 95% CI: 1.225–1.374, p<0.001), pre-procedural-shock (HR: 2.369, 95% CI: 2.201–2.549, p<0.001), pneumonia (HR: 1.883, 95% 1.761–2.014), and chronic skin ulcer (HR: 1.670, 95% 1.540–1.811). As shown in Supplementary Table 3, the claims-based predictors of long-term mortality used in the current study are similar to those defined in clinical studies.
Table 3.
Comparison of the c-statistics in each model
| C-statistic (95% CI) | IDI | IDI(p-value) | DeLong(p-value) | |
|---|---|---|---|---|
| Model 1 (Cardiac risk factors) | 0.60 (0.59–0.60) | − | − | − |
| Model 2 (Model 1 + non-cardiac risk factors) | 0.65 (0.64–0.65) | 0.032* | <0.001* | <0.001* |
| Model 3 (Model 2 + procedural characteristics) | 0.68 (0.67–0.68) | 0.019† | <0.001† | <0.001† |
| Model 4 (Model 3 + non-traditional frailty markers) | 0.70 (0.69–0.70) | 0.019‡ | <0.001‡ | <0.001‡ |
Model 2 vs Model 1,
Model 3 vs Model 2,
Model 4 vs Model 3.
IDI = integrated discrimination improvement; CI = confidence interval
DISCUSSION
In the present study, we show that administrative codes can be used to identify cardiac and non-cardiac risk factors, procedural characteristics, and markers of frailty which likely rendered TAVR patients at high surgical risk. The majority of TAVR patients in the database were frail, and the incorporation of these factors into statistical models improved the prediction of long-term mortality in patients undergoing TAVR when combined with traditional risk factors. Furthermore, our results demonstrate that the rate of long-term mortality after TAVR gradually increases with an increasing number of recorded non-traditional frailty markers. These findings illustrate the impact of frailty on outcomes for patients undergoing TAVR.
Impact of Frailty on Long-Term Mortality
There is a paucity of clinical studies for patients with structural heart disease which include an assessment of risk factors for frailty. For instance, the STS/ACC TVT Registry, the largest registry collecting patient characteristics and outcomes related to TAVR procedures in the US, collects only a few frailty markers such albumin, hemoglobin, and the 5-meter speed test 44. However, it has been demonstrated that incorporating frailty improves the prediction of mortality following TAVR. Adding a frailty index improved the c-statistic for discrimination of 1-year mortality of the STS-PROM score from 0.64 to 0.68 (p<0.001), and improved the discrimination of the logistic EuroSCORE from 0.67 to 0.72 (p<0.001) 31.
Afilalo et al. 19 reported that the average prevalence of frailty in the TAVR population, based on assessments using seven separate frailty scales, was 54% (37%−74%). Despite both its commonality and its importance, frailty scales are infrequently collected in routine clinical care 45. In the absence of prospectively collected data on frailty, we show prognostic information can be identified in claims data. Further, we showed that including claims-based frailty alongside traditional risk factors in statistical modeling significantly improved the discrimination of long-term mortality. However, in our landmark analysis, adding non-traditional frailty markers did not predict differences in perioperative mortality. These results suggest that the incorporation of frailty is more important for the prediction of long-term mortality than perioperative mortality. Additionally, the improvement performance of adding frailty was similar in magnitude to adding procedural covariates which are well-known risk factors 10 in the TAVR population. We believe that by merging claims-based frailty data with ongoing structural heart registries, we might enhance the ability to define patient risk and understand long-term outcomes, improve hospital benchmarking, and increase the completeness of data collection.
Predictors of All-cause Mortality After TAVR
Predictive models for in-hospital and 30-day mortality have provided moderate discrimination in the TVT registry (c-statistic: 0.67 in the training dataset; 0.66 in the validation sample) 10 and the France-2 Registry (c-statistic: 0.67 in the training sample; 0.59 in the validation sample) 16. The primary advantage of our analysis of MedPAR data is the inclusion of a large, generalized population with the ability to track mortality over the long term. Although we do not have all the necessary variables to calculate traditional surgical risk scores such as the STS-PROM or logistic EuroSCORE, the final model derived from MedPAR data had reasonable discrimination (c-statistic = 0.70), and the claims-based predictors of long-term mortality used in the current study are similar to those defined in clinical studies (Supplementary Table 3).
Limitations
There are several limitations to the present study. The indications and criteria for patient selection for TAVR are not available, and administrative coding may misclassify some comorbidities and complications compared with prospective collection using standard clinical trial definitions. Additionally, because the study population was limited to Medicare beneficiaries, we did not have information on all patients younger than 65 years of age who might have undergone TAVR in the US, and those patients < 65 who were included in the study may not be representative of younger patients overall.
CONCLUSIONS
Our findings show that risk prediction models that include frailty as identified in claims data can be used to more accurately predict long-term mortality risk after TAVR. Linkage to claims data may allow enhanced mortality risk prediction for studies that do not collect information on frailty.
Supplementary Material
What is Known:
Frailty and disability play a key role in the identification of older patients’ potential for improvement after transcatheter aortic valve replacement.
What the Study Adds:
Our findings show that the inclusion of frailty and disability markers as identified in Medicare beneficiaries significantly improved the prediction of long-term mortality.
These claims-based risk factors may allow for enhanced mortality prediction in the absence of prospectively collected data.
Acknowledgments
Sources of Funding: Members of the study team are supported by funding from the National Heart, Lung, and Blood Institute (1F32HL1407–11[J.B.S.], R01HS024520–01[C.S.] and 1R01HL136708–01[R.W.Y.]).
Footnotes
Disclosures: The following authors have no conflicts of interest to declare: JJP, DJC, JBS, LRV, DSP, and CS. Dr. Popma reports grants from Medtronic, Abbott Vascular, and Direct Flow Medical and personal fees from Boston Scientific, Cordis, and Direct Flow Medical, outside the submitted work. Dr. Yeh reports investigator-initiated grant funding from Abiomed, grant support from Boston Scientific, and consulting from Abbott, Medtronic, and Teleflex, outside the submitted work.
REFERENCES
- 1.Manning WJ. Asymptomatic aortic stenosis in the elderly: a clinical review. JAMA. 2013;310:1490–7. [DOI] [PubMed] [Google Scholar]
- 2.Nishimura RA, Otto CM, Bonow RO, Carabello BA,Erwin JP, Fleisher LA, Jneid H, Mack MJ, McLeod CJ, O’Gara PT, Rigolin VH, Sundt TM, Thompson A. 2017 AHA/ACC Focused Update of the 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2017;135:1159–95 [DOI] [PubMed] [Google Scholar]
- 3.Baumgartner H, Falk V, Bax JJ, Bonis MD, Hamm C, Holm PJ, Iung B, Lancellotti P, Lansac E, Muñoz DR, Rosenhek R, Sjögren J, Mas PT, Vahanian A, Walther T, Wendler O, Windecker S, Zamorano JL; ESC Scientific Document Group. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J. 2017;38:2739–91. [DOI] [PubMed] [Google Scholar]
- 4.Leon MB, Smith CR, Mack M, Miller DC, Moses JW, Svensson LG, Tuzcu EM, Webb JG, Fontana GP, Makkar RR. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med. 2010;363:1597–607. [DOI] [PubMed] [Google Scholar]
- 5.Adams DH, Popma JJ, Reardon MJ, Yakubov SJ, Coselli JS, Deeb GM, Gleason TG, Buchbinder M, Hermiller J, Kleiman NS, Chetcuti S, Heiser J, Merhi W, Zorn G, Tadros P, Robinson N, Petrossian G, Hughes GC, Harrison JK, Conte J, Maini B, Mumtaz M, Chenoweth S, JK Oh for the U.S. CoreValve Clinical Investigators. Transcatheter aortic-valve replacement with a self-expanding prosthesis. N Engl J Med. 2014;370:1790–8. [DOI] [PubMed] [Google Scholar]
- 6./>Smith CR, Leon MB, Mack MJ, Miller C, Moses JW, Svensson LG, Tuzcu EM, Webb JG, Fontana GP, Makkar RR, Williams M, Dewey T, Kapadia S, Babaliaros V, Thourani VH, Corso P, Pichard AD, Bavaria JE, Herrmann HC, Akin JJ, Anderson WN, Wang D, Pocock SJ, for the PARTNER Trial Investigators. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med. 2011;364:2187–98. [DOI] [PubMed] [Google Scholar]
- 7.Leon MB, Smith CR, Mack MJ, Makkar RR, Svensson LG, Kodali SK, Thourani VH, Tuzcu EM, Miller DC, Herrmann HC, Doshi D, Cohen DJ, Pichard AD, Kapadia S, Dewey T, Babaliaros V, Szeto WY, Williams MR, Kereiakes D, Zajarias A, Greason KL, Whisenant BK, Hodson RW, Moses JW, Trento A, Brown DL, Fearon WF, Pibarot P, Hahn RT, Jaber WA, Anderson WN, Alu MC, Webb JG. Transcatheter or Surgical Aortic-Valve Replacement in Intermediate-Risk Patients. N Engl J Med. 2016;374:1609–20. [DOI] [PubMed] [Google Scholar]
- 8.Reardon MJ, Van Mieghem NM, Popma JJ, Kleiman NS, Søndergaard L, Mumtaz M, Adams DH, Deeb GM, Maini B, Gada H, Chetcuti S, Gleason T, Heiser J, Lange R, Merhi W, Oh JK, Olsen PS, Piazza N, Williams M, Windecker S, Yakubov SJ, Grube E, Makkar R, Lee JS, Conte J, Vang E, Nguyen H, Chang Y, Mugglin AS, Serruys PW, Kappetein AP; SURTAVI Investigators. Surgical or transcatheter aortic-valve replacement in intermediate-risk patients. N Engl J Med. 2017;376:1321–31. [DOI] [PubMed] [Google Scholar]
- 9.O’Brien SM, Shahian DM, Filardo G, Ferraris VA, Haan CK, Rich JB, Normand SL, DeLong ER, Shewan CM, Dokholyan RS, Peterson ED, Edwards FH, Anderson RP. The Society of Thoracic Surgeons 2008 cardiac surgery risk models: part 2--isolated valve surgery. Ann Thorac Surg. 2009;88:23–42. [DOI] [PubMed] [Google Scholar]
- 10.Edwards FH, Cohen DJ, O’Brien SM, Peterson ED, Mack MJ, Shahian DM, Grover FL, Tuzcu EM, Thourani VH, Carroll J, Brennan JM, Brindis RG, Rumsfeld J, Holmes DR Jr. Development and validation of a risk prediction model for in-hospital mortality after transcatheter aortic valve replacement. JAMA Cardiol. 2016;1:46–52. [DOI] [PubMed] [Google Scholar]
- 11.Nashef SA, Roques F, Michel P, Gauducheau E, Lemeshow S, Salamon R. European system for cardiac operative risk evaluation (Euro SCORE). Eur J Cardiothorac Surg. 1999;16:9–13. [DOI] [PubMed] [Google Scholar]
- 12.de Brito FS Jr., Carvalho LA, Sarmento-Leite R, Mangione JA, Lemos P, Siciliano A, Caramori P, São Thiago L, Grube E, Abizaid A; Brazilian TAVI Registry investigators. Outcomes and predictors of mortality after transcatheter aortic valve implantation: results of the Brazilian registry. Catheter Cardiovasc Interv. 2015;85:153–62. [DOI] [PubMed] [Google Scholar]
- 13.Arnold SV, Reynolds MR, Lei Y, Magnuson EA, Kirtane AJ, Kodali SK, Zajarias A, Thourani VH, Green P, Rodés-Cabau J, Beohar N, Mack MJ, Leon MB, Cohen DJ; PARTNER Investigators. Predictors of poor outcomes after transcatheter aortic valve replacement: results from the PARTNER (Placement of Aortic Transcatheter Valve) trial. Circulation. 2014;129:2682–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baron SJ, Arnold SV, Wang K, Magnuson EA, Chinnakondepali K, Makkar R, Herrmann HC, Kodali S, Thourani VH, Kapadia S, Svensson L, Brown DL, Mack MJ, Smith CR, Leon MB, Cohen DJ; PARTNER 2 Investigators. Health Status Benefits of Transcatheter vs Surgical Aortic Valve Replacement in Patients With Severe Aortic Stenosis at Intermediate Surgical Risk: Results From the PARTNER 2 Randomized Clinical Trial. JAMA Cardiol. 2017;2:837–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mack MJ, Brennan JM, Brindis R, Carroll J, Edwards F, Grover F, Shahian D, Tuzcu EM, Peterson ED, Rumsfeld JS, Hewitt K, Shewan C, Michaels J, Christensen B, Christian A, O’Brien S, Holmes D; STS/ACC TVT Registry. Outcomes following transcatheter aortic valve replacement in the United States. JAMA. 2013;310:2069–77. [DOI] [PubMed] [Google Scholar]
- 16.Iung B, Laouénan C, Himbert D, Eltchaninoff H, Chevreul K, Donzeau-Gouge P, Fajadet J, Leprince P, Leguerrier A, Lièvre M, Prat A, Teiger E, Laskar M, Vahanian A, Gilard M; FRANCE 2 Investigators. Predictive factors of early mortality after transcatheter aortic valve implantation: individual risk assessment using a simple score. Heart. 2014;100:1016–23. [DOI] [PubMed] [Google Scholar]
- 17.Popma JJ, Adams DH, Reardon MJ, Yakubov SJ, Kleiman NS, Heimansohn D, Hermiller J Jr, Hughes GC, Harrison JK, Coselli J, Diez J, Kafi A, Schreiber T, Gleason TG, Conte J, Buchbinder M, Deeb GM, Carabello B, Serruys PW, Chenoweth S, Oh JK; CoreValve United States Clinical Investigators. Transcatheter aortic valve replacement using a self-expanding bioprosthesis in patients with severe aortic stenosis at extreme risk for surgery. J Am Coll Cardiol. 2014;63:1972–81. [DOI] [PubMed] [Google Scholar]
- 18.Hermiller JB, Yakubov SJ Jr., Reardon MJ, Deeb GM, Adams DH, Afilalo J, Huang J, Popma JJ; CoreValve United States Clinical Investigators. Predicting Early and Late Mortality After Transcatheter Aortic Valve Replacement. J Am Coll Cardiol. 2016;68:343–52. [DOI] [PubMed] [Google Scholar]
- 19.Afilalo J, Lauck S, Kim DH, Lefèvre T, Piazza N, Lachapelle K, Martucci G, Lamy A, Labinaz M, Peterson MD, Arora RC, Noiseux N, Rassi A, Palacios IF, Généreux P, Lindman BR, Asgar AW, Kim CA, Trnkus A, Morais JA, Langlois Y, Rudski LG, Morin JF, Popma JJ, Webb JG, Perrault LP. Frailty in older adults undergoing aortic valve replacement: the FRAILTY-AVR study. J Am Coll Cardiol. 2017;70:689–700. [DOI] [PubMed] [Google Scholar]
- 20.Green P, Woglom AE, Genereux P, Daneault B, Paradis JM, Schnell S, Hawkey M, Maurer MS, Kirtane AJ, Kodali S, Moses JW, Leon MB, Smith CR, Williams M. The impact of frailty status on survival after transcatheter aortic valve replacement in older adults with severe aortic stenosis: a single-center experience. JACC Cardiovasc Interv. 2012;5:974–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thongprayoon C, Cheungpasitporn W, Kashani K. The impact of frailty on mortality after transcatheter aortic valve replacement. Ann Transl Med. 2017;5:144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stortecky S, Schoenenberger AW, Moser A, Kalesan B, Jüni P, Carrel T, Bischoff S, Schoenenberger CM, Stuck AE, Windecker S, Wenaweser P. Evaluation of multidimensional geriatric assessment as a predictor of mortality and cardiovascular events after transcatheter aortic valve implantation. JACC: Cardiovasc Interv. 2012;5:489–96. [DOI] [PubMed] [Google Scholar]
- 23.Schoenenberger AW, Stortecky S, Neumann S, Moser A, Jüni P, Carrel T, Huber C, Gandon M, Bischoff S, Schoenenberger CM, Stuck AE, Windecker S, Wenaweser P. Predictors of functional decline in elderly patients undergoing transcatheter aortic valve implantation (TAVI). Eur Heart J 2013;34:684–92. [DOI] [PubMed] [Google Scholar]
- 24.Puls M, Sobisiak B, Bleckmann A, Jacobshagen C, Danner BC, Hünlich M, Beißbarth T, Schöndube F, Hasenfuß G, Seipelt R, Schillinger W. Impact of frailty on short- and long-term morbidity and mortality after transcatheter aortic valve implantation: risk assessment by Katz Index of activities of daily living. EuroIntervention 2014;10:609–19. [DOI] [PubMed] [Google Scholar]
- 25.Kim DH, Kim CA, Placide S, Lipsitz LA, Marcantonio ER. Preoperative frailty assessment and outcomes at 6 months or later in older adults undergoing cardiac surgical procedures: a systematic review. Ann Intern Med 2016;165: 650–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kleczynski P, Dziewierz A, Bagienski M, Rzeszutko L, Sorysz D, Trebacz J, Sobczynski R, Tomala M, Stapor M, Dudek D. Impact of frailty on mortality after transcatheter aortic valve implantation. Am Heart J 2017;185:52–8. [DOI] [PubMed] [Google Scholar]
- 27.Bureau ML, Liuu E, Christiaens L, Pilotto A, Mergy J, Bellarbre F, Ingrand P, Paccalin M; MPI_AGE Project Investigators. Using a multidimensional prognostic index (MPI) based on comprehensive geriatric assessment (CGA) to predict mortality in elderly undergoing transcatheter aortic valve implantation. Int J Cardiol 2017;236:381–6. [DOI] [PubMed] [Google Scholar]
- 28.Assmann P, Kievit P, van der Wulp K Verkroost M, Noyez L, Bor H, Schoon Y. Frailty is associated with delirium and mortality after transcatheter aortic valve implantation. Open Heart 2016;3:e000478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Okoh AK, Chauhan D, Kang N, Haik N, Merlo A, Cohen M, Haik B, Chen C, Russo MJ. The impact of frailty status on clinical and functional outcomes after transcatheter aortic valve replacement in nonagenarians with severe aortic stenosis. Catheter Cardiovasc Interv 2017;90:1000–6. [DOI] [PubMed] [Google Scholar]
- 30.Shimura T, Yamamoto M, Kano S, Kagase A, Kodama A, Koyama Y, Tsuchikane E, Suzuki T, Otsuka T, Kohsaka S, Tada N, Yamanaka F, Naganuma T, Araki M, Shirai S, Watanabe Y, Hayashida K; OCEAN-TAVI Investigators. Impact of the Clinical Frailty Scale on outcomes after transcatheter aortic valve replacement. Circulation 2017;135:2013–24. [DOI] [PubMed] [Google Scholar]
- 31.Schoenenberger AW, Moser A, Bertschi D, Wenaweser P, Windecker S, Carrel T, Stuck AE, Stortecky S. Improvement of Risk Prediction After Transcatheter Aortic Valve Replacement by Combining Frailty With Conventional Risk Scores. JACC: Cardiovasc Interv. 2018;11:395–403. [DOI] [PubMed] [Google Scholar]
- 32.Krumholz HM, Nuti SV, Downing NS, Normand SL, Wang Y. Mortality, Hospitalizations, and Expenditures for the Medicare Population Aged 65 Years or Older, 1999–2013. JAMA. 2015;314:355–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schermerhorn ML, Buck DB, O’Malley AJ, Curran T, McCallum JC, Darling J, Landon BE. Long-Term Outcomes of Abdominal Aortic Aneurysm in the Medicare Population. N Engl J Med. 2015;373:328–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schermerhorn ML, O’Malley AJ, Landon BE. Long-Term Outcomes of Abdominal Aortic Aneurysm Repair. N Engl J Med. 2015;373:2088–9. [DOI] [PubMed] [Google Scholar]
- 35.Kim DH, Schneeweiss S, Glynn RJ, Lipsitz LA, Rockwood K, Avorn J. Measuring Frailty in Medicare Data: Development and Validation of a Claims-Based Frailty Index. J Gerontol A Biol Sci Med Sci. 2017;73:980–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Faurot KR, Jonsson Funk M, Pate V, Brookhart MA, Patrick A, Hanson LC, Castillo WC, Stürmer T. Using claims data to predict dependency in activities of daily living as a proxy for frailty. Pharmacoepidemiol Drug Saf. 2015;24:59–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Segal JB, Chang HY, Du Y, D Walston J, C Carlson M, Varadhan R. Development of a claims-based frailty indicator anchored to a well-established frailty phenotype. Med Care 2017;55:716–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Segal JB, Huang J, Roth DL, Varadhan R. External validation of the claims-based frailty index in the national health and aging trends study cohort. Am J Epidemiol. 2017;186:745–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Coleman CI, Turpie AGG, Bunz TJ, Beyer-Westendorf J. Effectiveness and Safety of Rivaroxaban Versus Warfarin in Frail Patients with Venous Thromboembolism. Am J Med. 2018;131:933–8 [DOI] [PubMed] [Google Scholar]
- 40.Martinez BK, Sood NA, Bunz TJ, Coleman CI. Effectiveness and Safety of Apixaban, Dabigatran, and Rivaroxaban Versus Warfarin in Frail Patients With Nonvalvular Atrial Fibrillation. J Am Heart Assoc. 2018;7:e008643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fried LP, Tangen CM, Walston J. Cardiovascular Health Study Collaborative Research Group. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:146–57 [DOI] [PubMed] [Google Scholar]
- 42.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 1988;44:837–45. [PubMed] [Google Scholar]
- 43.Pencina MJ, D’Agostino RB, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27: 157–72. [DOI] [PubMed] [Google Scholar]
- 44.Grover FL, Vemulapalli S, Carroll JD, Edwards FH, Mack MJ, Thourani VH, Brindis RG, Shahian DM, Ruiz CE, Jacobs JP, Hanzel G, Bavaria JE, Tuzcu EM, Peterson ED, Fitzgerald S, Kourtis M, Michaels J, Christensen B, Seward WF, Hewitt K, Holmes DR Jr; STS/ACC TVT Registry. 2016 Annual Report of The Society of Thoracic Surgeons/American College of Cardiology Transcatheter Valve Therapy Registry. J Am Coll Cardiol. 2017;69:1215–30. [DOI] [PubMed] [Google Scholar]
- 45.Van Mieghem NM, Dumonteil N, Chieffo A, Roux Y, van der Boon RM, Giustino G, Hartman E, Aga Y, de Jong L, Abi Ghanem M, Marcheix B, Cavazza C, Carrié D, Colombo A, Kappetein AP, de Jaegere PP, Tchetche D. Current decision making and short-term outcome in patients with degenerative aortic stenosis: the Pooled-RotterdAm-Milano-Toulouse In Collaboration Aortic Stenosis survey. EuroIntervention. 2016;11:1305–13. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.