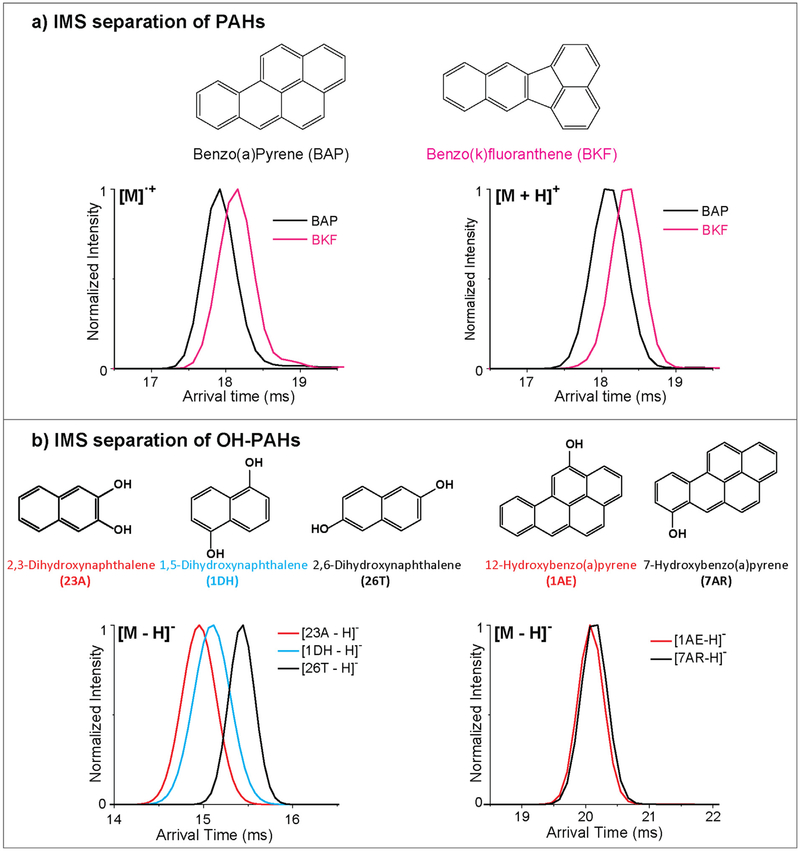
Fig. 4.
IMS separations of a) PAHs and b) OH-PAHs. a) IMS separations of the isomeric PAHs benzo(a)pyrene (BAP) and benzo(k)fluoranthene (BKF) in their radical and protonated forms illustrate BAP is smaller than BKF in both cases. Longer arrival times are observed for the protonated species of both PAHs, illustrating how the proton increases the CCS size.b) IMS separations of isomeric OH-PAHs illustrate a case where IMS is able to distinguish one set of depronated isomers and one case where it cannot. The chemical structure of each molecule is shown.