Abstract
Introduction
Cell sheets using myoblasts have been developed for the treatment of heart failure after myocardial infarction (MI) bridging to heart transplantation. Stem cells are supposed to be better than myoblasts as a source of cells, since they possess a potential to proliferate and differentiate into cardiomyocytes, and also have capacity to secrete angiogenic factors. Adipose-derived stem cells (ASCs) obtained from fat tissues are expected to be a new cell source for ASC sheet therapies. Administration of angiotensin II receptor blockers (ARBs) is a standard therapy for heart failure after MI. However, it is not known whether ARBs affect the cell sheet therapy. This study aimed to examine ameliorating effects of ASC sheets on heart failure and remodeling after MI, and how pretreatment with ARBs prior to the creation of MI and ASC sheet transplantation modifies the effects of ASC sheets.
Methods
ASCs were isolated from fat tissues of wild-type rats, and ASC sheets were engineered on temperature-responsive dishes. In in vitro studies using cultured cells, mRNA levels of vascular endothelial growth factor (VEGF) in ASCs were determined by RT-PCR in the presence of angiotensin II and/or an ARB, irbesartan, under normoxia and hypoxia; mRNA and protein levels of angiotensin II receptor type 1a (AT1aR), type 1b (AT1bR) and type 2 (AT2R) were also determined by RT-PCR and western blotting. In in vivo studies using a rat MI model, effects of transplanted ASC sheets and/or irbesartan on cardiac functions and remodeling after MI were evaluated by echocardiography, histological analysis and molecular biological techniques.
Results
In the in vitro studies, ASCs expressed higher levels of VEGF mRNA under hypoxia. They also expressed mRNA and protein of AT1aR but not AT1bR or AT2R. Under normoxia, angiotensin II increased the level of VEGF mRNA in ASCs, which was abolished by irbesartan. Under hypoxia, irbesartan reduced the level of VEGF mRNA in ASCs regardless of whether angiotensin II was present or not. In the in vivo studies, ASC sheets improved cardiac functions after MI, leading to decreased interstitial fibrosis and increased capillary density in border zones. These effects of ASC sheets were abolished by oral administration of irbesartan before MI and their transplantation.
Conclusions
ASC sheets ameliorated cardiac dysfunctions and remodeling after MI via increasing VEGF expression, which was abolished by pretreatment with irbesartan before the creation of MI and transplantation.
Keywords: Adipose-derived stem cell sheet, Myocardial infarction, Angiotensin II, Irbesartan, VEGF
Abbreviations: ANP, atrial natriuretic peptide; ARB, angiotensin receptor blocker; ASC, adipose-derived stem cell; AT1(2)R, angiotensin II receptor type 1(2); CRT, cardiac resynchronization therapy; EF, ejection fraction; FGF, fibroblast growth factor; FS, fractional shortening; HGF, hepatocyte growth factor; LVEDD, left ventricular end-diastolic diameter; LVESD, left ventricular end-systolic diameter; MI, myocardial infarction; MSC, mesenchymal stem cell; RAS, renin–angiotensin system; VEGF, vascular endothelial growth factor; vWF, von Willebrand factor
Highlights
-
•
ASC sheets ameliorated cardiac dysfunctions and remodeling after MI in rats.
-
•
ASC sheets could protect hearts after MI through activation of AT1aR.
-
•
Pretreatment with an ARB, irbesartan, abolished ameliorating effects of ASC sheets.
-
•
We need to pay attention to the attenuating effect of ARBs on cell sheet therapy.
1. Introduction
Arteriosclerotic diseases such as myocardial infarction (MI) are the major cardiovascular problems associated with the westernization of Japanese lifestyle. Treatment of choice for acute MI is percutaneous coronary angioplasty or surgical bypass operation, both of which have decreased mortality and morbidity of patients with MI. Oxidative stress following MI causes cardiac remodeling to lead to ischemic heart failure; cardiac protective medicines that decrease oxidative stress are utilized against the ischemic heart failure, but do not sufficiently increase the survival rate of patients.
The renin–angiotensin system (RAS), as well as the sympathetic nervous system, has been reported to be activated in MI. These neurohumoral factors are involved in cardiac remodeling (e.g., cardiac hypertrophy, cell death and fibrosis), which leads to terminal heart failure [1]. To prevent the RAS from exaggerated activation, angiotensin II receptor blockers (ARBs) are used as the first-line medicine. However, with developing the drug resistant heart failure, either cardiac resynchronization therapy (CRT) or auxiliary artificial heart is applied, and eventually heart transplantation is conducted to cure the patients with terminal heart failure. Since the donor for heart transplantation is lacking, it is necessary to reduce the number of patients to whom heart transplantation should be applied. Therefore, an alternative way to bridge between medicine and heart transplantation is needed.
Cell-based regenerative medicine could improve blood supply to the damaged heart, and could minimize both the area of infarction and cardiac remodeling [2], [3], [4] through secretions of several cytokines such as hepatocyte growth factor (HGF) and vascular endothelial growth factor (VEGF) [5]. Direct injection of cells into heart is problematic because of a significant loss of live cells and proarrhythmic effects [6], [7]. Tissue engineering using cell sheets has been developed to overcome these disadvantages; the cell sheet could have improved viability of cells [8], [9] and prolonged secretion of cytokines [10]. Initially, sheets from skeletal myoblast cells were reported to improve cardiac functions in patients with severe heart failure [11]. However, collecting skeletal myoblasts from patients requires invasive procedures, and it takes a long time to obtain a sufficient number of myoblasts. In addition, myoblasts secrete low levels of cytokines and lack capability to differentiate into cardiomyocytes. Thus, an alternative source of stem cells for cell sheets is required.
Adipose-derived stem cells (ASCs) belong to the mesenchymal stem cells (MSCs). It has been reported that they secrete several cytokines such as VEGF, HGF, and fibroblast growth factor (FGF), and prevent cardiac dysfunction and remodeling after MI [12], [13], [14] by inducing neovascularization [15], [16], [17], [18].
A local RAS is reported to operate both in hearts and in MSCs [19]. Long-term activation of the RAS induces progressive injury to hearts, leading to hypertrophy, fibrosis and inflammation [20]. It has also been reported that activation of the RAS could play a pivotal role in the secretion of VEGF by MSCs derived from bone marrow [21]; exposure of the MSCs to angiotensin II increased expression of VEGF mRNA, which could be suppressed by ARBs [22]. In addition, MSCs treated with ARBs have been reported to improve the efficiency of cardiomyogenic transdifferentiation and improve cardiac functions via angiogenesis [23].
Since ARBs are the first-line medicine for the treatment of patients who suffer from MI, cell sheet transplantation might be combined with administration of ARBs in the future. It has been reported that ARBs such as candesartan administered after the creation of MI significantly improve the function of hearts transplanted with MSCs after MI [23]. However, it has never been tested whether pretreatment with ARBs before the creation of MI and ASC sheet transplantation affects ASC sheet-induced improvement of cardiac functions after MI. In the present study, we evaluated effects of the pretreatment with an ARB, irbesartan, on functions of rat hearts transplanted with ASC sheets after MI.
2. Materials and methods
2.1. In vitro study
2.1.1. Engineering of ASC sheets
ASCs were enzymatically isolated from the inguinal subcutaneous fat tissue of rats and cultured as previously described [15]. To prepare cell sheets, ASCs (2–3 passage) were cultured on 35-mm temperature-responsive culture dishes (2 × 105/cm2) (UpCell, Cell Seed Inc., Tokyo, Japan) in an incubator at 37 °C. After the temperature-responsible culture dish was preincubated with PBS for 24 h, 1 × 106 cells were transferred on a dish, cultured for 48 h, and then maintained at 20 °C for 1 h to release them as an intact sheet.
2.1.2. Real-time RT-PCR analysis
ASC sheets were incubated at 37 °C under normal (21% O2) or hypoxic (<2% O2) conditions for 48 h using an AnaeroPack system (MITSUBISHI GAS CHEMICAL Inc., Tokyo, Japan). Total RNA was extracted from ASC sheets using RNeasy Mini kit (QIAGEN Inc., Valencia, CA, USA). Real-time RT-PCR analysis of HGF, VEGF, basic FGF (bFGF), hypoxia inducible factor-1α (HIF-1α), angiotensin II receptor type 1a (AT1aR), 1b (AT1bR) and 2 (AT2R), and β-actin was performed using 1 μg RNA with the ABI 7900HT Fast Real-Time PCR System (Applied Biosystems, Foster City, CA, USA). The primers shown in Table 1, Table 2 were designed to amplify their genomic regions, as described elsewhere [15]. Their mRNA levels were expressed as ratios to those of β-actin. The mRNA levels of VEGF, HGF, HIF-1α and bFGF as ratios to that of β-action were further normalized to the control MI group values, and the ratio of VEGF mRNA under hypoxia was also given as 2−ΔΔCt using the comparative CT method.
Table 1.
PCR primers for angiogenic factors and beta-actin.
| Gene name | Probe# | Sequence |
|---|---|---|
| Rattus β-actin | 115 | Forward: 5′-CTAAGGCCAACCGTGAAAAG-3′ |
| Reverse: 5′-GCCTGGATGGCTACGTACA-3′ | ||
| Rattus VEGF | 4 | Forward: 5′-TTAAACGAACGTACTTGCAGATG-3′ |
| Reverse: 5′-TCTAGTTCCCGAAACCCTGA-3′ | ||
| Rattus HGF | 49 | Forward: 5′-GATTGGATCAGGACCTTGTGA-3′ |
| Reverse: 5′-CCATTCTCATTTTGTGTTGTTCA-3′ | ||
| Rattus bFGF | 7 | Forward: 5′-TCTTCCTGCGCATCCATC-3′ |
| Reverse: 5′-GCTTGGAGCTGTAGTTTGACG-3′ |
Table 2.
PCR primers for angiotensin II receptors.
| Gene name | Sequence |
|---|---|
| Rattus AT1aR | Forward: 5′-AGAGTCAGGAGCTGGATGGA-3′ |
| Reverse: 5′-ACAAAGGTTCCTTGCCCTTT-3′ | |
| Rattus AT1bR | Forward: 5′-CAAAAGGAGATGGGAGGTCA-3′ |
| Reverse: 5′-TTCAGGCAAGCTGTTCTGTG-3′ | |
| Rattus AT2R | Forward: 5′-TTCCCTTCCATGTTCTGACC-3′ |
| Reverse: 5′-TGGAGCCAAGTAATGGGAAC-3′ |
2.1.3. Western blotting
ASCs were harvested and lysed by sonication in a buffer PBS supplemented with 1% polyoxyethylene octylphenyl ether (Triton X-100), 0.5% sodium deoxycholate, 0.1% SDS, 1.5 mM aprotinin, 21 mM leupeptin, 15 mM pepstain and 1 mM phenylmethylsulfonyl fluoride. Proteins were separated on SDS-PAGE and electrotransferred to PVDF membranes. The membranes were blocked with 5% non-fat dry milk in PBS plus 0.1% Tween and immunoblotted with primary antibodies against AT1R [sc-579, Santa Cruz Biotechnology, Dallas, TX, USA] and AT2R [sc-9040, Santa Cruz]. Rabbit anti-AT1R and AT2R antibodies were used as the 1st antibody with 800-fold dilution by PBS. The blots were developed by using an ECL system (Amersham, Biosciences, Piscataway, NJ, USA).
2.2. In vivo study
2.2.1. Applications of cell sheets and irbesartan to a rat MI model
Adult male syngeneic Lewis rats (200–250 g), obtained from Japan SLC, Inc. (Hamamatsu, Japan), served as cell donors and recipients. The experimental protocols were approved by the Institutional Animal Care and Use Committee, Faculty of Medicine, Tottori University. Rats were randomly assigned to 4 groups: (1) neither ASC sheet transplantation nor irbesartan treatment (control MI group, n = 5); (2) ASC sheet transplantation alone (ST group, n = 5); (3) irbesartan pretreatment alone (Irb group, n = 6); and (4) ASC sheet transplantation with irbesartan pretreatment (ST + Irb group, n = 4). For all the groups, acute MI was created by ligation of the left anterior descending artery, as previously described [18]. Irbesartan at 30 mg/kg/day was orally administered to the Irb and ST + Irb group rats for 6 weeks (from 7 days prior to the creation of MI to 5 weeks after MI). ASC sheet transplantation was performed for the ST and ST + Irb group rats 7 days after the creation of MI. We confirmed that transplanted cell sheets existed on the surface of hearts by luminescent signals of ASCs derived from transgenic rats harboring the luciferase gene (data not shown). Details of the experimental protocol are shown in Supplemental Fig. S1.
2.2.2. In vivo measurements of blood pressure and heart rate
Systolic blood pressure (SBP), diastolic blood pressure (DBP) and heart rate (HR) were measured by a tail cuff system (BP-98A, Softron, Tokyo, Japan) a week prior to MI operation, and 2 and 4 weeks after ASC sheet transplantation (3 and 5 weeks after MI).
2.2.3. Echocardiography for assessment of left ventricular functions
Cardiac function was evaluated by echocardiography 7 days before ASC sheet transplantation (just before MI), and 2 and 4 weeks after ASC sheet transplantation (3 and 5 weeks after MI) using a 12-MHz transducer (LOGIQ P5J and 12L, GE Healthcare, Fairfield, CT, USA). Short-axis two-dimensional images at the mid-papillary level of the left ventricle were stored as digital loops. The end-systolic and end-diastolic cavity areas were determined by tracing the endocardial borders to calculate the fractional area shortening (FS). Left ventricular internal diameters at end-systole (LVESD) and end-diastole (LVEDD) were measured between the mitral valve and papillary muscles to calculate the ejection fraction (EF). All measurements were made thrice and averaged by two independent experienced examiners in a blinded fashion.
2.2.4. Measurement of plasma atrial natriuretic peptide (ANP) levels
Blood samples were obtained from the femoral vein just before MI and 4 weeks after ASC sheet transplantation (5 weeks after MI). Plasma ANP concentrations were measured by ANP ELISA Kit (Assaypro, Saint Charles, MO, USA) in SRL laboratory.
2.2.5. Histopathological study
Four weeks after transplantation (5 weeks after MI), hearts were dissected, fixed in 10% formalin, and embedded in paraffin. The paraffin sections were subjected to hematoxylin–eosin staining, Masson's trichrome staining, and immunostaining with anti-von Willebrand factor (vWF) antibody. Masson's trichrome staining was performed to detect interstitial fibrosis. Transverse sections were obtained from the lateral wall, posterior wall, and septum. Two fields per section were analyzed by a microscope (BZ9000, Keyence, Osaka, Japan). Each field was scanned, and digital images were analyzed by Keyence analysis software. The paraffin sections were stained with an antibody against vWF (Abcam plc, Cambridge, UK) to detect capillaries in the border zone between the myocardium and scar. Capillaries were counted by light microscopy in two fields of transverse sections per animal. The number of capillaries in each field was averaged and expressed as capillary density.
2.3. Data analysis
All data are expressed as mean ± SEM. Bonferroni multiple comparison test was used for multiple group comparisons. A probability value of <0.05 was considered significant.
3. Results
3.1. Effects of hypoxia on expression of angiogenic factor mRNAs by ASCs in vitro
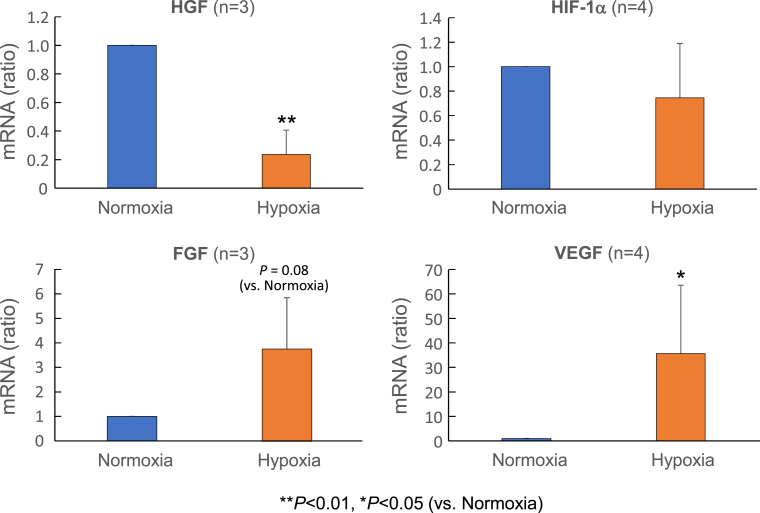
We measured angiogenic factor mRNAs in ASCs after exposure to normoxic or hypoxic conditions for 48 h (Fig. 1). Levels of VEGF mRNA were significantly higher under hypoxia than under normoxia. Levels of FGF mRNA trended to increase under hypoxia, whereas those of HGF mRNA significantly decreased. There was no significant difference in HIF-1α mRNA levels between normoxia and hypoxia.
Fig. 1.
mRNA levels of HGF, HIF-1α, FGF and VEGF in ASCs under normoxia and hypoxia conditions. Semi-quantitative RT-PCR analysis was performed to determine HGF, HIF-1α, FGF and VEGF mRNA expression levels relative to β-actin mRNA levels, which are expressed as the ratio to the control values under normoxia. The numbers of experiments are given in the parenthesis. *P < 0.05, **P < 0.01 (vs. Normoxia).
3.2. Irbesartan suppressed expression of VEGF mRNA in ASCs in vitro
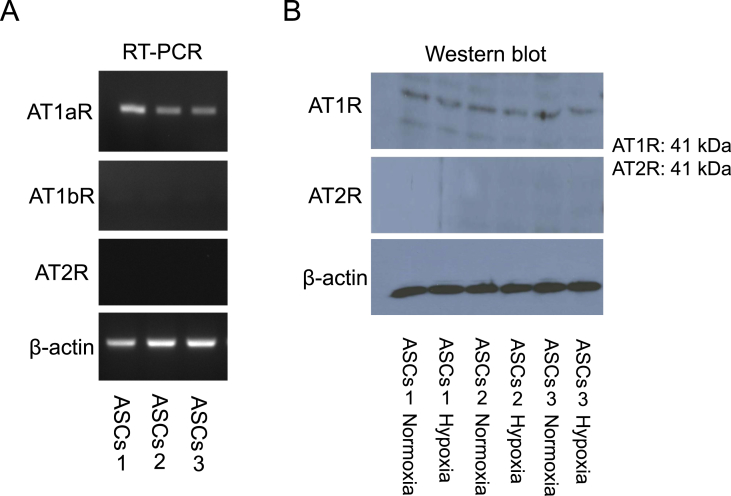
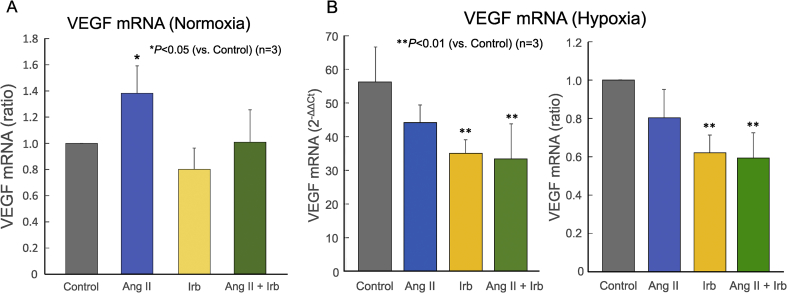
Angiotensin II increased the expression of VEGF mRNA in MSCs derived from bone marrow, which could be suppressed by ARBs [22]. Since it has been reported that the angiotensin receptor type 1 (AT1R) was expressed in MSCs, we studied the expression profiles of AT1R and AT2R in ASCs (Fig. 2). ASCs expressed AT1aR mRNA, but not AT1bR or AT2R mRNA, under normoxia (Fig. 2A); they expressed AT1aR protein alone both under normoxia and under hypoxia (Fig. 2B). Under normoxia, angiotensin II significantly increased the expression of VEGF mRNA in ASCs as well; irbesartan in itself did not alter the level of VEGF mRNA, but prevented angiotensin II from increasing VEGF mRNA (Fig. 3A). Under hypoxia, irbesartan significantly reduced the level of VEGF mRNA regardless of whether angiotensin II is present or absent, while angiotensin II did not alter the VEGF mRNA level (Fig. 3B).
Fig. 2.
mRNA and protein expressions of AT1aR, AT1bR and AT2R as well as β-actin in ASCs. A: Expressions of AT1aR, AT1bR and AT2R mRNAs in ASCs were determined by RT-PCR. B: Expression levels of AT1R (AT1aR) and AT2R proteins in ASCs were determined by western blot.
Fig. 3.
Effects of angiotensin II and/or irbesartan on VEGF mRNA expression in ASCs under normoxia (A) and hypoxia (B). Real-time RT-PCR analysis was performed for determination of VEGF mRNA expression levels relative to those of β-actin mRNA, which are expressed as the ratio to the control value (Control, gray bar) for ASCs treated with 100 nM angiotensin II alone (AngII, blue bar), 200 nM irbesartan alone (Irb, yellow bar), and both 100 nM angiotensin II and 200 nM irbesartan (AngII + Irb, green bar) (n = 3 each). **P < 0.01, *P < 0.05 (vs. Control).
3.3. Irbesartan ameliorated cardiac dysfunctions but abolished beneficial effects of ASC sheets in a rat MI model
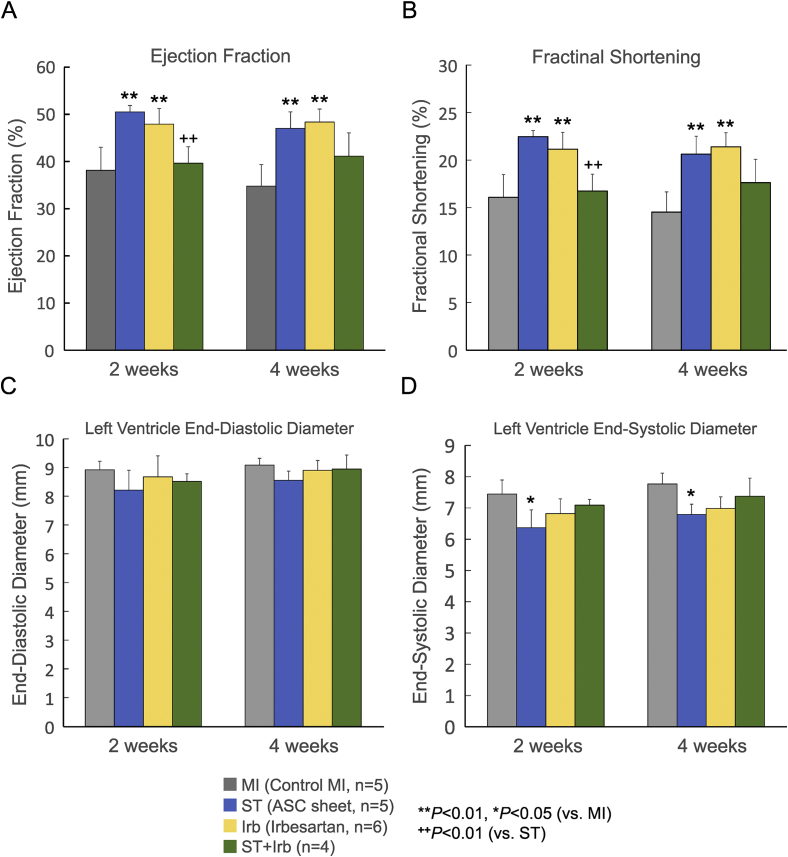
ARBs are administered to patients with MI in order to restore cardiac functions and attenuate cardiac remodeling. Here, we evaluated effects of ASC sheets and/or irbesartan on cardiac functions and remodeling after MI in vivo (Fig. 4). Lewis rats were assigned to 4 groups, i.e., the control MI group, ST group, Irb group and ST + Irb group, as described in the Methods section. EF was significantly lower in the control MI group than in sham-operated non-MI group (data not shown). EF in the ST group and Irb group, but not in the ST + Irb group, was significantly higher than that in the control MI group 2 and 4 weeks after ASC sheet transplantation (Fig. 4A). FS was significantly improved in the ST and Irb groups 2 and 4 weeks after transplantation, while not improved in the ST + Irb group (Fig. 4B). EF and FS were significantly lower in the ST + Irb group than in the ST group 2 weeks after transplantation. There was no significant difference in LVEDD among the 4 groups (Fig. 4C). LVESD, determined 2 and 4 weeks after transplantation, was significantly smaller in the ST group than in the control MI and other groups (Fig. 4D). Whereas irbesartan in itself did not influence LVESD, the agent prevented ASC sheets from decreasing LVESD.
Fig. 4.
Effects of ASC sheet transplantation and/or treatment with irbesartan on cardiac functions after MI. Ejection fraction (A), fractional area shortening (B), left ventricle end-diastolic diameter (C), and left ventricle end-systolic diameter (D) were measured by echocardiography 3 and 5 weeks after MI for the control MI hearts (MI, gray bars), and those treated with an ASC sheet alone (ST, blue bars), irbesartan alone (Irb, yellow bars) and both an ASC sheet and irbesartan (ST + Irb, green bars). The numbers of experiments are given in the parentheses (n = 4–6). *P < 0.05, **P < 0.01 (vs. control MI); ++P < 0.01 (vs. ST).
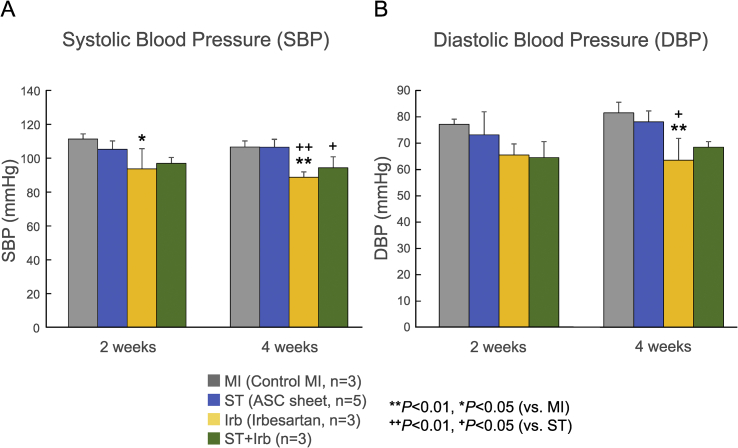
SBP and DBP in the Irb group were significantly lower than those of the control MI and ST groups 4 weeks after transplantation (5 weeks after MI), while those in the ST + Irb group were comparable to the control group values (Fig. 5). The plasma level of ANP measured 4 weeks after transplantation tended to be lower in the ST and Irb groups than in the control MI group, while comparable in the control MI and ST + Irb groups (Supplemental Fig. S2).
Fig. 5.
Effects of ASC sheet transplantation and/or treatment with irbesartan on systolic and diastolic blood pressures after MI. Systolic (A) and diastolic (B) blood pressures were measured 3 and 5 weeks after MI (2 and 4 weeks after ASC sheet transplantation) for the control MI hearts (MI, gray bars), and those treated with an ASC sheet alone (ST, blue bars), irbesartan alone (Irb, yellow bars) and both an ASC sheet and irbesartan (ST + Irb, green bars). The numbers of experiments are given in the parentheses (n = 3–5). *P < 0.05, **P < 0.01 (vs. control MI); ++P < 0.01, +P < 0.05 (vs. ST).
3.4. Irbesartan abolished the beneficial effects of ASC sheet on cardiac fibrosis and neovascularization after MI
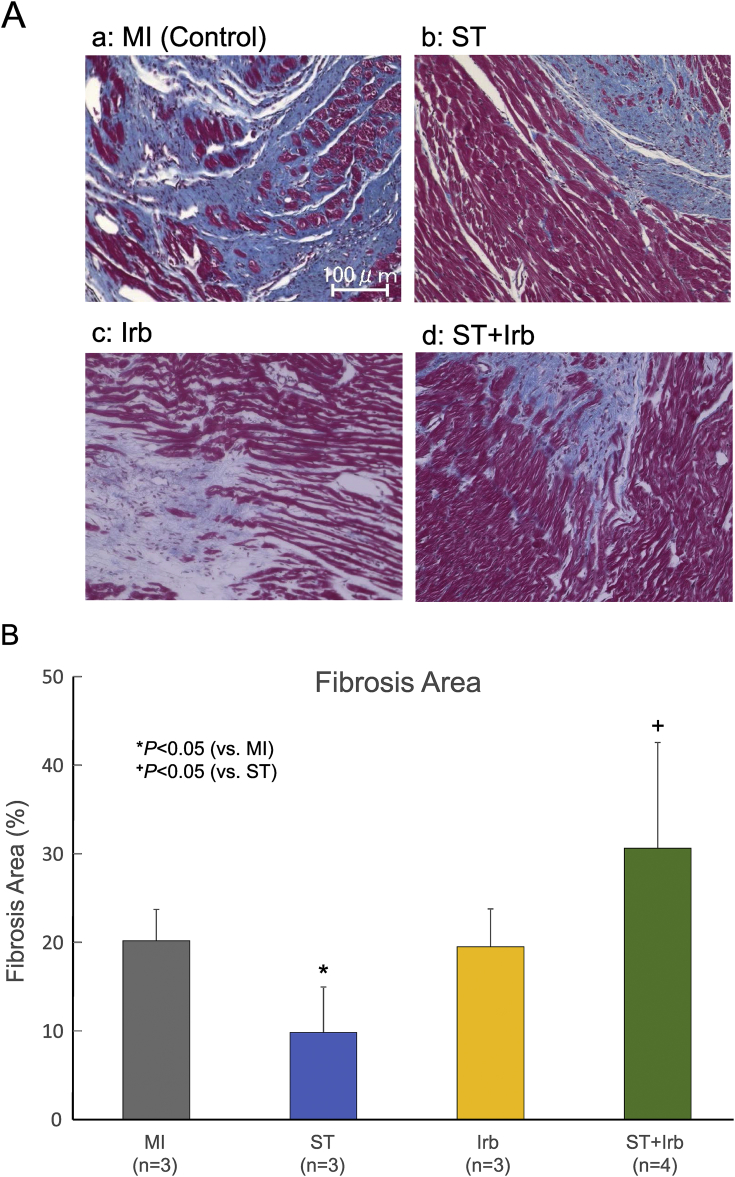
Cardiac fibrosis and neovascularization were evaluated by histological analysis 4 weeks after transplantation (Fig. 6, Fig. 7). In the control MI group, extensive fibrosis occurred around the infarcted region. This fibrosis remarkably reduced in the ST group, but not in the ST + Irb group (Fig. 6A). Fig. 6B shows the summary data obtained from multiple experiments (n = 3–4). The extent of cardiac fibrosis was significantly lower in the ST group than in the control MI group; this decrease of fibrosis did not occur in the Irb group or ST + Irb group, with the ST + Irb group fibrosis significantly greater than the ST group fibrosis.
Fig. 6.
Effects of ASC sheet transplantation and/or treatment with irbesartan on interstitial fibrosis in remote zone of MI hearts. A: Representative pictures of interstitial fibrosis in MI hearts from the control MI (a), ST (b), Irb (c) and ST + Irb (d) groups 4 weeks after transplantation. Picrosirius-red staining was performed to detect interstitial fibrosis. Scale bar = 100 μm. B: The summary of the prevalence of interstitial fibrosis in the remote zone of MI hearts from the 4 groups. The numbers of experiments are given in the parentheses for each group (n = 3–4). *P < 0.05 (vs. control MI), +P < 0.05 (vs. ST).
Fig. 7.
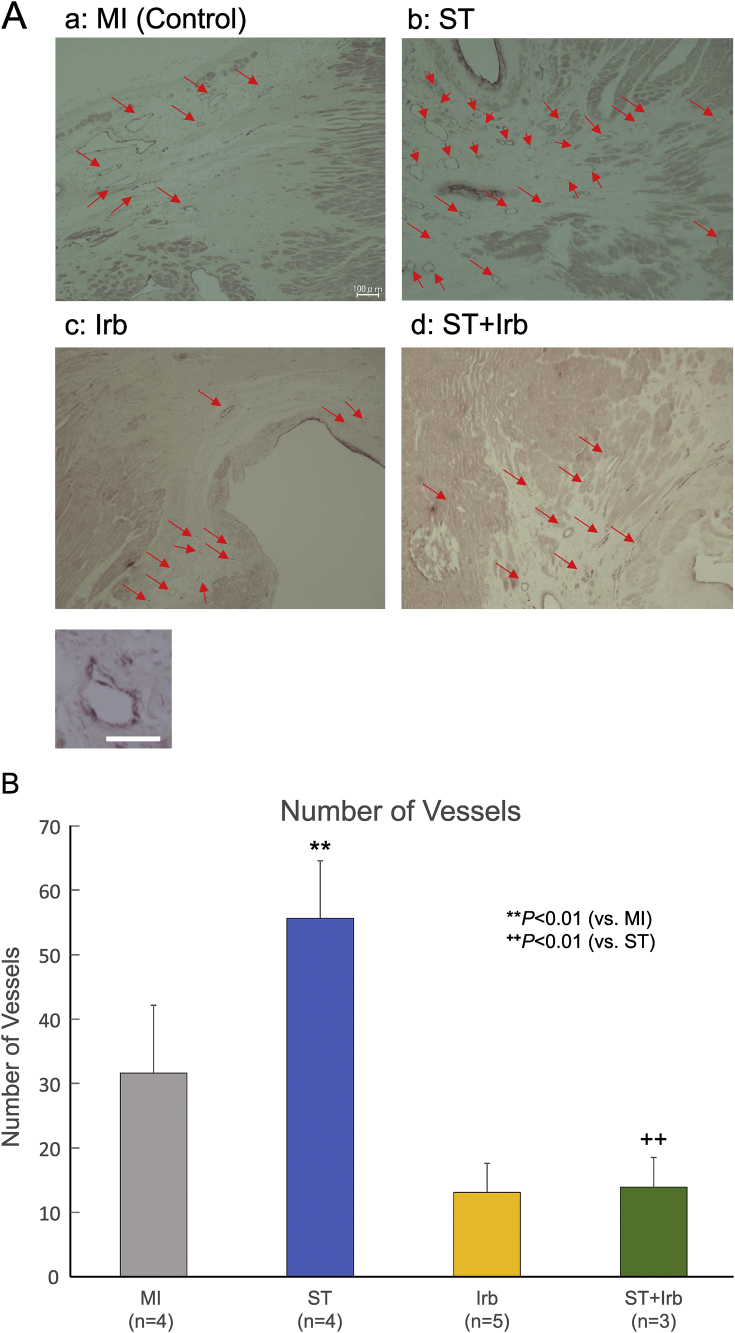
Effects of ASC sheet transplantation and/or treatment with irbesartan on neovascularization in the border zone of MI hearts. A: Representative pictures of capillary vessels stained with an antibody against von Willebrand factor (vWF) in the border zone of MI hearts from the control MI (a), ST (b), Irb (c) and ST + Irb (d) groups 5 weeks after MI (red arrows; scale bar = 100 μm). The inset shows an expanded scale view of a capillary vessel stained with the antibody against vWF (scale bar = 25 μm). B: The summary of the numbers of vWF-positive vessels in the border zone of MI hearts from each group. The number of capillary vessels was determined in 5 randomly selected fields per animal, and expressed as an averaged value. The numbers of experiments are given in the parentheses. **P < 0.01 (vs. control MI), ++P < 0.01 (vs. ST).
The capillary density in the ST group was remarkably increased, as compared with those in the other groups (Fig. 7A). Fig. 7B shows the summary data on capillary density obtained from multiple experiments (n = 4–5). The capillary density was significantly higher in the ST group than in the control MI group, but was lower in the ST + Irb group than in the ST group.
4. Discussion
In the present study, we found in in vitro and in vivo studies that (1) the expression of VEGF mRNA in ASCs was augmented by hypoxia (Fig. 1), (2) ASCs expressed AT1aR, but not AT1bR or AT2R (Fig. 2), (3) angiotensin II increased expression of VEGF mRNA in ASCs under normoxia with this effect abolished by irbesartan (Fig. 3A), (4) under hypoxia, irbesartan reduced VEGF mRNA expression of ASCs in the absence and presence of angiotensin II (Fig. 3B), (5) preventive actions of transplanted ASC sheets on cardiac dysfunctions after MI could be abolished by pretreatment with irbesartan prior to the creation of MI and transplantation (Fig. 4), and (6) both suppression of fibrosis and enhancement of neovascularization induced by ASC sheets were abolished by the pretreatment with irbesartan (Fig. 6, Fig. 7).
In the present study, ASC sheets improved cardiac functions after MI. This beneficial effect might be attributable to increases in capillary density and reduction of fibrosis in MI hearts. Especially, LVESD was decreased by transplantation of ASC sheets in the ST group, which may be due to the improvement of contractility. Although we did not determine its underlying mechanism, our previous report [18] indicates that ASC sheets enhance contractions of MI hearts, which may partially explain the reduction of LVESD in the ST group.
Angiotensin II activates AT1R and promotes inflammation, hypertrophy and fibrosis. It is also known to enhance the expression of matrixins and increase the volume of the extracellular matrix [24]. It has been reported that the local RAS is expressed in the heart and MSCs [19]. Long-term activation of the RAS leads to the cardiac hypertrophy, fibrosis and inflammation [20], which are well known to be suppressed by pretreatment with ARBs.
Irbesartan has been reported to have cardioprotective actions in the Dahl salt-sensitive rat model [25] and MI models [26]; it reduced infiltration of macrophages and attenuated the increments of end-systolic and end-diastolic volumes of the hearts after MI [27]. In the present study, irbesartan in itself significantly improved cardiac functions after MI of Lewis rats. Irbesartan selectively blocks AT1R with slow dissociation from the receptor [28]; thus, the agent could block angiotensin II signaling by inhibition of AT1R, which can explain the potency of this agent in improving cardiac functions after MI. We found that blood pressures of the Irb group rats were significantly lower than those of the control MI group; the decreased afterload may contribute to the irbesartan-induced improvement of EF and FS without changes in fibrosis and angiogenesis of MI hearts.
In the present study, the pretreatment with irbesartan did not inhibit fibrosis after MI, while improved cardiac functions. However, it was reported that posttreatment with ARBs after MI decreased cardiac fibrosis in a rat MI model [26]. This discrepancy may be explained by the difference in experimental conditions, i.e., “pretreatment with ARB before MI” (in this study) vs. “posttreatment with ARB after MI” (in the previous study). MSCs pretreated with angiotensin II significantly suppressed fibrosis after MI [29]; the action of angiotensin II before and/or just after MI may also be necessary for the inhibition of fibrosis in MI hearts. We need further investigations to clarify whether posttreatment with RAS inhibitors such as irbesartan after MI inhibits fibrosis in our MI model. As described above, irbesartan could improve the cardiac function without reducing cardiac fibrosis after MI probably via decreasing the afterload.
The most prominent finding in the present study was that pretreatment with irbesartan prior to the creation of MI and ASC sheet transplantation abolished the ameliorating effects of ASC sheets on cardiac dysfunction and remodeling after MI. This suggests that the cardioprotective actions of ASC sheets involve a signaling via the RAS. Angiotensin II in itself has been shown to have beneficial effects on secretion of angiogenic factors from MSCs through activation of AT1R [21]. Exposure of MSCs derived from bone marrow to angiotensin II has been reported to increase their expression of VEGF mRNA through ERK1/2 and Akt pathways via AT1aR, which could be suppressed by ARBs [21], [22]. Angiotensin II applied to MSCs suppressed their apoptosis under hypoxia, increased VEGF secretion, accelerated tube formation in a co-culture with vascular endothelial cells, and facilitated the formation of gap junctions with cardiac myocytes accompanied by increases in the protein level of Cx43 [29]. Furthermore, injections of MSCs pretreated with angiotensin II into the ischemic region of MI hearts significantly improved the cardiac function and suppressed fibrosis by promoting angiogenesis [29]. It is well recognized that ASC sheets could restore the cardiac function after MI via activation of angiogenic factors such as VEGF [18]. In the present study, we found that ASCs expressed mRNA and protein of AT1aR, and treatment with angiotensin II significantly increased VEGF mRNA in ASCs under normoxia (but not under hypoxia); however, in the presence of irbesartan, angiotensin II did not increase VEGF mRNA under normoxia (or hypoxia), consistent with the notion that angiotensin II drives VEGF synthesis via activation of AT1aR [30]. Under hypoxia, irbesartan reduced VEGF mRNA expression enhanced by hypoxia, which also confirmed the previous report [22]. Taken together, irbesartan should suppress the angiotensin II and hypoxia-induced enhancement of VEGF synthesis in ASCs by blocking AT1aR, which may explain the in vivo data partially. However, we did not determine VEGF mRNA levels in the ASC sheets transplanted into MI hearts or phosphorylation of VEGF receptors in MI heart cells in the in vivo study; thus, further studies will be necessary to elucidate how the pretreatment with irbesartan could eliminate the protective effects of ASC sheets on the heart after MI. We have previously reported that ASC sheets significantly increase VEGF and HGF mRNA in the transplanted heart after MI while not affecting bFGF mRNA [18], suggesting that the pretreatment with irbesartan affects the mRNA levels of the angiogenic factors in the heart transplanted with ASC sheets after MI.
While it remains unclear in the present study how the pretreatment with irbesartan could abolish the ASC sheet-induced cardioprotective actions after MI, it also remains unelucidated how ASC sheet transplantation abolished the ameliorating action of the pretreatment with irbesartan on the dysfunction of MI hearts (Fig. 4A and B). Irbesartan caused the significant decrease in the blood pressure of MI rats (Fig. 5), which is well known to contribute to the improvement of EF and FS. However, the blood pressure in the ST + Irb group was not significantly lower than that in the control MI group; non-decreasing afterload may eliminate the improvements of EF and FS induced by irbesartan alone via decreasing the blood pressure. Nevertheless, it is unclear why the blood pressure was higher in the ST + Irb group than in the Irb group, and thus how ASC sheet transplantation inhibits the cardioprotective effects of irbesartan administered before (and after) MI remains to be elucidated.
Despite that the precise mechanism underlying the eliminating effect of the pretreatment with the ARB on ASC sheet-induced cardioprotection after MI is unknown, the clinical implication of this study may be clear. ASC sheets would be applicable to the drug-refractory patients for bridging to the heart transplantation. The pretreatment with the ARB abolished the preventive effects of ASC sheets on cardiac dysfunction and remodeling after MI, indicating that we have to pay attention to the period and timing of administration of ARBs before and after ASC sheet transplantation. We cannot necessarily apply the present results to the clinical setting directly, because the effects of ARBs on MI hearts and ASC sheet therapy may depend on the method of their administration: RAS inhibitors such as ACE inhibitors significantly improved the prognosis of MI patients when administered more than 24 h after MI, but not when administered immediately after MI [31]. These results suggest that irbesartan should not be administered to patients before or immediately after MI. We need further investigations on whether RAS inhibitors such as irbesartan administered 24 h or later after MI (posttreatment with the agents) inhibit cardioprotective actions of ASC sheet transplantation.
MI damages just before ASC sheet transplantation (1 week after MI) should be almost the same in the 4 groups to assess the ameliorating effects of ASC sheets on the MI damage accurately. In the present study, however, it was impossible to confirm that the MI damage was comparable among the 4 groups, since we aimed to evaluate the effects of oral administration of irbesartan before MI (before ASC sheet transplantation) on the function of hearts transplanted with ASC sheets after MI, and the pretreatment with irbesartan might affect the myocardial damage by MI.
5. Conclusion
We investigated the effects of ASC sheet transplantation and/or administration of the ARB irbesartan on cardiac functions and remodeling after MI in rat hearts. ASC sheets restored the cardiac function and ameliorated remodeling after MI through activation of AT1aR. Pretreatment with irbesartan abolished these ameliorating effects of ASC sheets. Thus, we should pay attention to the attenuating effect of ARBs on cell sheet therapy for the treatment of MI patients to whom ARBs are administered before and/or after MI.
Acknowledgements
Irbesartan was kindly gifted from Sumitomo Dainippon Pharma Co. Ltd. under approval by the pharmaceutical company Sanofi.
Footnotes
Peer review under responsibility of the Japanese Society for Regenerative Medicine.
Supplementary data related to this article can be found at https://doi.org/10.1016/j.reth.2018.08.005.
Any potential conflicts of interest
Dr. I. Hisatome reported receiving lecturer's fee from Sanwa Kagaku Kenkyusho Co. Ltd., Feizer Co. Ltd. and Fuji Yakuhin Co. Ltd., and research grants from Dainippon Sumitomo Pharma Co. Ltd., Teijin Pharma, Fuji Yakuhin Co. Ltd. and Sanwa Kagaku Kenkyusho Co. Ltd.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
Fig. S1: A schema of the experimental protocol for the creation of MI, ASC sheet transplantation, administration of irbesartan, and evaluations of cardiac functions and remodeling. To the Irb and ST + Irb group rats, irbesartan at 30 mg/kg/day was orally administered for 7 days before the creation of MI and for 5 weeks after MI. Seven days after MI, the ST and ST + Irb group rats underwent ASC sheet transplantation. Cardiac functions were determined by echocardiography 1, 3 and 5 weeks after MI (just after the transplantation, and 2 and 4 weeks after the transplantation for the ST and ST + Irb groups). Interstitial fibrosis, neovascularization and serum ANP levels were evaluated 5 weeks after MI (4 weeks after the transplantation).
Fig. S2: Serum levels of ANP in the control MI rats (MI, gray bar), and MI rats transplanted with an ASC sheet (ST, blue bar), treated with irbesartan (Irb, yellow bar), and received both ASC sheet transplantation and irbesartan treatment (ST + Irb, green bar). ANP levels were measured 5 weeks after MI (4 weeks after transplantation).
References
- 1.Leosco D., Rengo G., Iaccarino G., Golino L., Marchese M., Fortunato F. Exercise promotes angiogenesis and improves beta-adrenergic receptor signalling in the post-ischaemic failing rat heart. Cardiovasc Res. 2008;78:385–394. doi: 10.1093/cvr/cvm109. [DOI] [PubMed] [Google Scholar]
- 2.Kawamura M., Miyagawa S., Miki K., Saito A., Fukushima S., Higuchi T. Feasibility, safety, and therapeutic efficacy of human induced pluripotent stem cell-derived cardiomyocyte sheets in a porcine ischemic cardiomyopathy model. Circulation. 2012;126:S29–S37. doi: 10.1161/CIRCULATIONAHA.111.084343. [DOI] [PubMed] [Google Scholar]
- 3.Menasché P., Alfieri O., Janssens S., McKenna W., Reichenspurner H., Trinquart L. The Myoblast Autologous Grafting in Ischemic Cardiomyopathy (MAGIC) trial: first randomized placebo-controlled study of myoblast transplantation. Circulation. 2008;117:1189–1200. doi: 10.1161/CIRCULATIONAHA.107.734103. [DOI] [PubMed] [Google Scholar]
- 4.Bolli R., Tang X.L., Sanganalmath S.K., Rimoldi O., Mosna F., Abdel-Latif A. Intracoronary delivery of autologous cardiac stem cells improves cardiac function in a porcine model of chronic ischemic cardiomyopathy. Circulation. 2013;128:122–131. doi: 10.1161/CIRCULATIONAHA.112.001075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhao T., Zhao W., Meng W., Liu C., Chen Y., Gerling I.C. VEGF-C/VEGFR-3 pathway promotes myocyte hypertrophy and survival in the infarcted myocardium. Am J Transl Res. 2015;7:697–709. [PMC free article] [PubMed] [Google Scholar]
- 6.Raedschelders K., Ansley D.M., Chen D.D. The cellular and molecular origin of reactive oxygen species generation during myocardial ischemia and reperfusion. Pharmacol Ther. 2012;133:230–255. doi: 10.1016/j.pharmthera.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 7.Song H., Cha M.J., Song B.W., Kim I.K., Chang W., Lim S. Reactive oxygen species inhibit adhesion of mesenchymal stem cells implanted into ischemic myocardium via interference of focal adhesion complex. Stem Cells. 2010;28:555–563. doi: 10.1002/stem.302. [DOI] [PubMed] [Google Scholar]
- 8.Hamdi H., Planat-Benard V., Bel A., Puymirat E., Geha R., Pidial L. Epicardial adipose stem cell sheets results in greater post-infarction survival than intramyocardial injections. Cardiovasc Res. 2011;91:483–491. doi: 10.1093/cvr/cvr099. [DOI] [PubMed] [Google Scholar]
- 9.Pätilä T., Miyagawa S., Imanishi Y., Fukushima S., Siltanen A., Mervaala E. Comparison of arrhythmogenicity and proinflammatory activity induced by intramyocardial or epicardial myoblast sheet delivery in a rat model of ischemic heart failure. PLoS One. 2015;10:e0123963. doi: 10.1371/journal.pone.0123963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Memon I.A., Sawa Y., Fukushima N., Matsumiya G., Miyagawa S., Taketani S. Repair of impaired myocardium by means of implantation of engineered autologous myoblast sheets. J Thorac Cardiovasc Surg. 2005;130:1333–1341. doi: 10.1016/j.jtcvs.2005.07.023. [DOI] [PubMed] [Google Scholar]
- 11.Miyagawa S., Saito A., Sakaguchi T., Yoshikawa Y., Yamauchi T., Imanishi Y. Impaired myocardium regeneration with skeletal cell sheets – a preclinical trial for tissue-engineered regeneration therapy. Transplantation. 2010;90:364–372. doi: 10.1097/TP.0b013e3181e6f201. [DOI] [PubMed] [Google Scholar]
- 12.Rehman J., Traktuev D., Li J., Merfeld-Clauss S., Temm-Grove C.J., Bovenkerk J.E. Secretion of angiogenic and antiapoptotic factors by human adipose stromal cells. Circulation. 2004;109:1292–1298. doi: 10.1161/01.CIR.0000121425.42966.F1. [DOI] [PubMed] [Google Scholar]
- 13.Sekiya N., Matsumiya G., Miyagawa S., Saito A., Shimizu T., Okano T. Layered implantation of myoblast sheets attenuates adverse cardiac remodeling of the infarcted heart. J Thorac Cardiovasc Surg. 2009;138:985–993. doi: 10.1016/j.jtcvs.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 14.Gnecchi M., Zhang Z., Ni A., Dzau V.J. Paracrine mechanisms in adult stem cell signaling and therapy. Circ Res. 2008;103:1204–1219. doi: 10.1161/CIRCRESAHA.108.176826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harada Y., Yamamoto Y., Tsujimoto S., Matsugami H., Yoshida A., Hisatome I. Transplantation of freshly isolated adipose tissue-derived regenerative cells enhances angiogenesis in a murine model of hind limb ischemia. Biomed Res. 2013;34:23–29. doi: 10.2220/biomedres.34.23. [DOI] [PubMed] [Google Scholar]
- 16.Miranville A., Heeschen C., Sengenès C., Curat C.A., Busse R., Bouloumié A. Improvement of postnatal neovascularization by human adipose tissue-derived stem cells. Circulation. 2004;110:349–355. doi: 10.1161/01.CIR.0000135466.16823.D0. [DOI] [PubMed] [Google Scholar]
- 17.Nakagami H., Maeda K., Morishita R., Iguchi S., Nishikawa T., Takami Y. Novel autologous cell therapy in ischemic limb disease through growth factor secretion by cultured adipose tissue-derived stromal cells. Arterioscler Thromb Vasc Biol. 2005;25:2542–2547. doi: 10.1161/01.ATV.0000190701.92007.6d. [DOI] [PubMed] [Google Scholar]
- 18.Otsuki Y., Nakamura Y., Harada S., Yamamoto Y., Ogino K., Morikawa K. Adipose stem cell sheets improved cardiac function in the rat myocardial infarction, but did not alter cardiac contractile responses to β-adrenergic stimulation. Biomed Res. 2015;36:11–19. doi: 10.2220/biomedres.36.11. [DOI] [PubMed] [Google Scholar]
- 19.Strawn W.B., Richmond R.S., Ann Tallant E., Gallagher P.E., Ferrario C.M. Renin–angiotensin system expression in rat bone marrow haematopoietic and stromal cells. Br J Haematol. 2004;126:120–126. doi: 10.1111/j.1365-2141.2004.04998.x. [DOI] [PubMed] [Google Scholar]
- 20.Xu J., Carretero O.A., Lin C.X., Cavasin M.A., Shesely E.G., Yang J.J. Role of cardiac overexpression of ANG II in the regulation of cardiac function and remodeling postmyocardial infarction. Am J Physiol Heart Circ Physiol. 2007;293:H1900–H1907. doi: 10.1152/ajpheart.00379.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shi R.Z., Wang J.C., Huang S.H., Wang X.J., Li Q.P. Angiotensin II induces vascular endothelial growth factor synthesis in mesenchymal stem cells. Exp Cell Res. 2009;315:10–15. doi: 10.1016/j.yexcr.2008.09.024. [DOI] [PubMed] [Google Scholar]
- 22.Fan Y., Wang L., Liu C., Zhu H., Zhou L., Wang Y. Local renin–angiotensin system regulates hypoxia-induced vascular endothelial growth factor synthesis in mesenchymal stem cells. Int J Clin Exp Pathol. 2015;8:2505–2514. [PMC free article] [PubMed] [Google Scholar]
- 23.Numasawa Y., Kimura T., Miyoshi S., Nishiyama N., Hida N., Tsuji H. Treatment of human mesenchymal stem cells with angiotensin receptor blocker improved efficiency of cardiomyogenic transdifferentiation and improved cardiac function via angiogenesis. Stem Cells. 2011;29:1405–1414. doi: 10.1002/stem.691. [DOI] [PubMed] [Google Scholar]
- 24.Siddesha J.M., Valente A.J., Sakamuri S.S., Yoshida T., Gardner J.D., Somanna N. Angiotensin II stimulates cardiac fibroblast migration via the differential regulation of matrixins and RECK. J Mol Cell Cardiol. 2013;65:9–18. doi: 10.1016/j.yjmcc.2013.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nako H., Kataoka K., Koibuchi N., Dong Y.F., Toyama K., Yamamoto E. Novel mechanism of angiotensin II-induced cardiac injury in hypertensive rats: the critical role of ASK1 and VEGF. Hypertens Res. 2012;35:194–200. doi: 10.1038/hr.2011.175. [DOI] [PubMed] [Google Scholar]
- 26.Watanabe R., Suzuki J., Wakayama K., Kumagai H., Ikeda Y., Akazawa H. Angiotensin II receptor blocker irbesartan attenuates cardiac dysfunction induced by myocardial infarction in the presence of renal failure. Hypertens Res. 2016;39:237–244. doi: 10.1038/hr.2015.141. [DOI] [PubMed] [Google Scholar]
- 27.Jugdutt B.I., Menon V. Upregulation of angiotensin II type 2 receptor and limitation of myocardial stunning by angiotensin II type 1 receptor blockers during reperfused myocardial infarction in the rat. J Cardiovasc Pharmacol Ther. 2003;8:217–226. doi: 10.1177/107424840300800307. [DOI] [PubMed] [Google Scholar]
- 28.Brunner H.R. The new angiotensin II receptor antagonist, irbesartan: pharmacokinetic and pharmacodynamic considerations. Am J Hypertens. 1997;10:311S–317S. doi: 10.1016/s0895-7061(97)00391-9. [DOI] [PubMed] [Google Scholar]
- 29.Liu C., Fan Y., Zhou L., Zhu H.Y., Song Y.C., Hu L. Pretreatment of mesenchymal stem cells with angiotensin II enhances paracrine effects, angiogenesis, gap junction formation and therapeutic efficacy for myocardial infarction. Int J Cardiol. 2015;188:22–32. doi: 10.1016/j.ijcard.2015.03.425. [DOI] [PubMed] [Google Scholar]
- 30.Liu C., Zhang J.-W., Hu L., Song Y.-C., Zhou L., Fan Y. Activation of the AT1R/HIF-1α/ACE axis mediates angiotensin II-induced VEGF synthesis in mesenchymal stem cells. Biomed Res Int. 2014;2014:627380. doi: 10.1155/2014/627380. Epub 2014 Oct 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Swedberg K., Held P., Kjekshus J., Rasmussen K., Rydén L., Wedel H. Effects of the early administration of enalapril on mortality in patients with acute myocardial infarction; results of the cooperative new Scandinavian enalapril survival study II (CONSENSUS II) N Engl J Med. 1992;327:678–684. doi: 10.1056/NEJM199209033271002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.