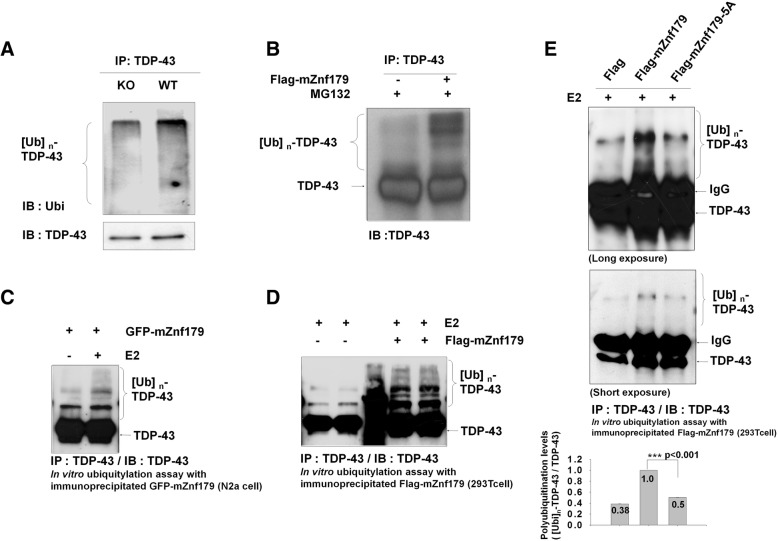
Fig. 5.
Znf179-mediates TDP-43 polyubiquitination in vitro and in vivo. a and b The total brain lysates of wild-type or Znf179-knockout mice (a) or 293 T cells transfected with Flag-mZnf179 and treated with 10 μM MG132 for 4 h (b) were immunoprecipitated by anti-TDP-43 antibody and analyzed by Western blotting with anti-ubiquitin or anti-TDP-43 antibodies. c, d and e For in vitro polyubiquitination assays, endogenous TDP-43 that was immunoprecipitated with anti-TDP-43 antibody from non-treated N2a cells lysates was included in a mixture of purified E1, ubiquitin, Mg2+-ATP, UbcH5c E2 conjugating enzyme, and the E3 ligase, Znf179. The Znf179 E3 ligase was immunoprecipitated from N2a cells stably expressing GFP-mZnf179 (c) or from 293 T cells transiently transfected with Flag-mZnf179 or with Flag-mZnf179-5A mutant (d and e). The ubiquitination levels of TDP-43 were analyzed by anti-TDP-43 antibody. Data were presented as the mean ± SEM (n = 3) (*** p < 0.001, groups were compared by t-test, two-tailed p values). The polyubiquitination levels of TDP-43 ([Ubi]n-TDP-43 / TDP-43) were compared between Flag-mZnf179 and Flag-mZnf179-5A groups (e)