Abstract
Objective:
Using screening mammography, this study investigated the association between obesity and axillary lymph node (LN) size and morphology.
Methods:
We conducted a retrospective review of 188 females who underwent screening mammography at an academic medical centre. Length and width of the LN and hilum were measured in the largest, mammographically visible axillary node. The hilo-cortical ratio (HCR) was calculated as the hilar width divided by the cortical width. Measurements were performed by a board certified breast radiologist and a resident radiology physician. Inter-rater agreement was assessed with Pearson correlation coefficient. We performed multivariable regression analysis for associations of LN measurements with body mass index (BMI), breast density and age.
Results:
There was a strong association between BMI and LN dimensions, hilum dimensions and HCR (p < 0.001 for all metrics). There was no significant change in cortex width with increasing BMI (p = 0.15). Increases in LN length and width were found with increasing BMI [0.6 mm increase in length per unit BMI, 95% CI (0.4–0.8), p < 0.001 and0.3 mm increase in width per unit BMI, 95% CI(0.2–0.4), p < 0.001, respectively]. Inter-rater reliability for lymph node and hilum measurements was 0.57–0.72.
Conclusion:
We found a highly significant association between increasing BMI and axillary LN dimensions independent of age and breast density with strong interobserver agreement. The increase in LN size was driven by expansion of the LN hilum secondary to fat infiltration.
Advances in knowledge:
This preliminary work determined a relationship between fat infiltrated axillary lymph nodes and obesity.
Introduction
Ectopic fat deposition within organs triggers structural, metabolic and inflammatory changes that contribute to obesity-related pathology.1–4 Axillary lymph nodes are organelles that demonstrate variable size and architecture on mammograms due to marked differences in the degree of fat-infiltration of the lymph node hilum (Figure 1). Ectopic adipose tissue is associated with an increased risk of cardio-metabolic disease in the obese population and ectopic fat deposition within organs may lead to organ dysfunction.1–7 Obesity is associated with impairment of the lymphatic system; however, the underlying mechanisms are unclear. Considering the known adverse effects of ectopic fat in other organs, fat-infiltrated lymph nodes may play a role in obesity-related lymphatic and immune system pathology.
Figure 1.
Screening mammogram of a 60-year-old female with BMI of 34: Left MLO view. (a) Left (MLO) view demonstrates a combination of normal (thin white arrows) and fat-infiltrated (open white arrow) axillary lymph nodes. (b) Magnified view of fat-infiltrated node Left MLO view with measurement axes as follows: total node length (solid black line), hilum length (dashed black double arrow), total node width (solid white line), hilum width (dashed white double arrow) and cortical width (solid white double arrow).MLO,medial lateral oblique.
The high utilization of screening mammography in up to 85% of age-eligible females in the UnitedStates and Europe provides an opportunity to study fat deposition within axillary lymph nodes.8, 9 It is generally accepted that fat-infiltrated nodes are more commonly seen on mammograms of obese females and older females; however, to our knowledge no studies have established an association between obesity and mammographically visualized, fat-infiltrated nodes. The aim of our study was to evaluate the relationship between lymph node (LN) morphology and body mass index (BMI).
MethodsandMaterials
An institutional review board approved this retrospective study. Screening mammograms from randomly selected days between January 2014 and June 2015 were chosen, resulting in 293 patients for initial review. Inclusion required BMI data in the medical record; exclusion criteria included history of breast cancer, breast biopsy in the past 3 years, and history of breast surgery. These criteria yielded 188 patients for analysis. Data were collected from the electronic medical record (Epic Systems Corporation, Verona, WI). Obesity was defined as BMI 30, overweight as BMI < 30 and25, and normal weight as BMI <25.
Two-dimensional full field digital mammograms were obtained with Selenia and Dimensions Hologic units (Hologic Incorporated, Bedford, MA). Images were reviewed on Barco 3-megapixel MDCG-3221 monitors (Kortrijk, Belgium) with Philips PACS v.3.6 (Philips Healthcare; Best, Netherlands). Independent LN measurements while blinded to patient history were performed by a breast radiologist with 14 years of experience (RDA) and a junior radiology resident (SJH). For each patient, the medial lateral oblique views were reviewed for visible axillary lymph nodes. The single largest LN within either axilla was chosen as the index node and was included for analysis if at least 80% of the node was visible. The LN cortex was defined as the radiodense peripheral portion of the node, and the LN hilum was measured as the radiolucent central area (Figure 1). The hilo-cortical ratio (HCR) was defined as the width of the radiolucent fatty hilum divided by the width of the mammographically dense cortex.
We evaluated the association of LN dimensions and hilum dimensions, breast density, and age with BMI, using independent samples t-test and the Fishers exact test. Multivariable linear regression models assessed the independent associations of LN dimensions (length and width) with BMI, adjusting for age and breast density. Inter-rater reliability between the two readers was assessed with Pearson correlation coefficient using a standard interpretation of strength of association. The measurements reported in the manuscript are the measurements of reader 1 (RDA). Statistical analysis was performed with IBM SPSS v. 22 (Armonk, New York, NY).
Results
Axillary lymph nodes were visible in 78% of patients (145/188) and were more commonly visualized in females with higher BMI (Table 1). The overall lymph node dimensions, hilum dimensions and HCR increased significantly with increasing BMI from normal to obese (p < 0.001 for these measurements for both readers, RDA and SJH. Reader RDA's measurements areshownin Table 1andSJHmeasurementsareshowninSupplementary Table 1. However, cortex width was not associated with BMI (p = 0.15, Table 1). There was good to strong inter-rater agreement for most measurements with Pearson correlation coefficient of 0.64 for lymph node length, 0.57 for hilum length, 0.72 for LN width, 0.68 for hilum width and 0.40 for cortex width. In 10/11 (91%) cases with discrepant measurements between readers, different nodes were chosen as the index node for analysis when multiple large nodes were present (Figure 2). Nonetheless, both sets of LN and hilar measurements were significantly associated with obesity (p < .001) despite lack of agreement.
Table 1. Association between bodymassindex(BMI), lymph node dimensions and clinical characteristics
| Characteristics | BMI | |||
| Normal weight | Over-weight | Obese | p | |
| Patients (N) | 75 | 55 | 58 | |
| Age | 59 years (10) | 57 years (10) | 59 years (10) | 0.49 |
| Fatty or scattered breast density | 25 (33%) | 31 (56%) | 48 (83%) | <0.001 |
| Patients with visible lymph node | 51 (68%) | 45 (82%) | 50 (86%) | 0.03 |
| Lymph node length | 14 mm (6) | 16 mm (7) | 23 mm (8) | <0.001 |
| Lymph node width | 8 mm (3) | 9 mm (3) | 12 mm (3) | <0.001 |
| Hilum length | 9 mm (5) | 11 mm (7) | 18 mm (8) | <0.001 |
| Hilum width | 4 mm (3) | 5 mm (3) | 9 mm (4) | <0.001 |
| Cortex Width | 2.5 mm (1.0) | 2.7 mm (1.2) | 3.0 mm (1.3) | 0.15 |
| Hilo-cortical ratio | 2.0 (1.5) | 2.2 (1.1) | 3.5 (2.2) | <0.001 |
| Lymph node length 20 mm | 6 (12%) | 7 (16%) | 33 (66%) | <0.001 |
| Hilo-cortical ratio 2.0 | 16 (31%) | 22 (49%) | 37 (74%) | <0.001 |
Values in the table are reported as N (%) or mean (standard deviation).
Normal weight is BMI <25. Overweight is BMI 25 and<30. Obese is BMI 30.
Figure 2.
Screening mammogram of a 47-year-old female with BMI of 30: Superior portion bilateral MLO views. Bilateral fat infiltrated nodes in which reader one measured the largest axillary LN in the right axilla (black double arrow) and reader two measured the largest axillary node in the left axilla (white double arrow). Dimensions were discrepant from each other, yet both sets of measurements were highly significantly associated with obesity (p <0.001).MLO,mediallateraloblique.
We found that a one-unit increase in BMI was associated with increased LN length and width [0.6 mm increase in LN length per unit BMI, 95% CI (0.4–0.8), p < 0.001 and 0.3 mm increase in LN width per unit BMI, 95% CI (0.2–0.4), p < 0.001, respectively], after adjusting for age and breast density. There was a strong association between increasing hilum length and increasing lymph node length (R2 = 0.90). Similarly, we found a strong association between the widths of the hilum and lymph node (R2 = 0.80). In contrast, there was no association between LN cortex width and width of the entire lymph node (R2 = 0.09).
Discussion
Our study demonstrates a significant association between increased BMI and increased fat accumulation within the LN hilum that is independent of patient age and breast density. LN and hilar length, LN and hilar width, and HCR increased with increasing BMI; and this change was due to an increase in the size of the fatty hilum without any significant change in the width of the parenchymal cortex (Figure 1). The enlargement of the LN is therefore driven by hilar fat deposition. There is strong interobserver agreement for lymph node and hilar width and modest interobserver agreement for LN and hilar length. Of note, despite modest inter-rater agreement for some measurements, all of these measurements were independently significantly associated with obesity for both readers (p < 0.001). This was in part due to the selection of a different index LN by each reader when multiple nodes were present (Figure 2). Although measurements varied when a different index node was chosen by each reader, significance was maintained for both readers. Strong interobserver agreement for several measurements and independent highly significant measures of diverse index nodes suggests that these findings are reproducible.
The width of the cortex was the measurement with the lowest inter-rater correlation, and there may be several reasons for this. The cortical width is the smallest dimension measured, usually within the range of 2–4 mm. Small variations in measurement may lead to bigger discrepancies relative to the absolute width thereby resulting in apparent decreased agreement among readers. Of interest, the cortex appears to be thinner and more effaced in fat-infiltrated nodes than in normal nodes; however, this apparent difference was not significant in our study, and a larger sample size may be necessary to demonstrate this effect (Figure 3). Our study suggests that fat infiltrated nodes may represent a biomarker of obesity on screening mammography, a highly utilized screening examination in the UnitedStates and Europe.
Figure 3.
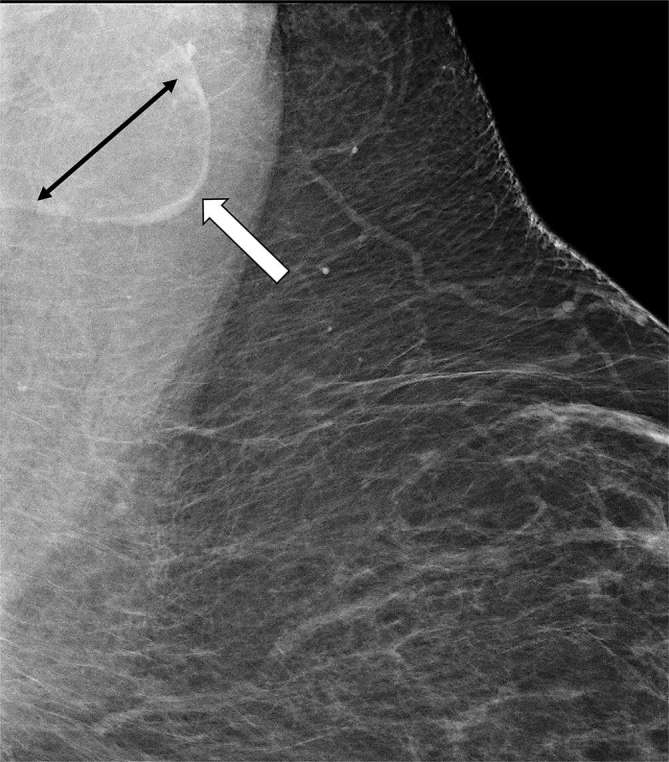
Screening mammogram of a 42-year-old female with BMI of 47: Magnified superior left MLO view. Thin, effaced cortex (white arrow) around expanded radiolucent fat-infiltrated hilum (black double arrow). Thinned cortex may be due to cortical atrophy or may represent a stable volume of cortical tissue stretched over a markedly enlarged hilum.BMI,bodymassindex;MLO,mediallateraloblique.
Additional study of mammographically visualized fat infiltrated nodes may also help to elucidate mechanisms of immune system dysfunction in obese patients. Although there is no known relationship between LN fat accumulation and immune or lymphatic dysfunction, it is known that obesity is complicated by an increased risk of infections, an increased risk of mortality due to infectious processes, and impaired lymphatic transport.10, 11 Several animal studies suggest that, similar to fat infiltration of other organs, fat infiltration of the lymphatic system may be associated with altered host immune response.12, 13 Ectopic fat accumulation impairs organ function in several ways, including compression of functioning tissue and secretion of inflammatory compounds.2 Adipose expansion of the central hilum could impair nodal function in a comparable manner: by exerting mass effect on the surrounding cortex, and by the secretion of pro-inflammatory cytokines triggering local or systemic inflammatory reactions.
Our study evaluates the morphology of axillary lymph nodes on mammograms obtained to screen for breast cancer. Axillary lymph nodes represent the watershed drainage for breast tissue, and the status of the axilla is the most powerful indicator of long-term survival in breast cancer patients. Obese females are at increased risk for breast cancer, and they have increased mortality secondary to advanced stage at the time of presentation compared to non-obese females.14 Proposed mechanisms contributing to poor outcomes in obese patients with breast cancer include increased breast epithelial and tumour cell proliferation stimulated by hyperinsulinemia, and tumour growth fueled by abundant fat substrate within the microenvironment.15 Peritumoral breast fat has recently been shown to be associated with axillary LN involvement in obese patients with early breast cancer.16 Similar mechanisms could be at play within fat-infiltrated axillary nodes whereby excess hilar fat may fuel the growth of micro-metastases. In addition, fat-infiltrated nodes may have altered immune function that impairs their ability to control early metastatic deposits. A better understanding of the role of increased axillary LN fat in obese females may therefore improve our understanding of poor outcomes in obese patients with breast cancer.
Our study demonstrates that enlarged, fat-infiltrated lymph nodes are associated with increased BMI independent of patient age and breast density, and that this association is due to expansion of the LN hilum by radiolucent adipose tissue. If these preliminary findings are validated in larger studies, fat-infiltrated axillary nodes visualized on screening mammograms could serve as a surrogate biomarker of obesity thereby prompting additional screening for obesity related pathology and serving as a foundation for future investigation of the role of fat infiltrated nodesin cardio-metabolic disease, immune dysfunction and poor breast cancer outcomes in obese females.
FUNDING
Dr diFlorio-Alexander and Dr Onega were partially funded by a National Cancer Institute-funded Breast Cancer Surveillance Consortium grant (P01CA154292). Dr MacKenzie was partially funded by the Dartmouth Clinical and Translational Science Institute (NIH award number UL1TR001086).
ACKNOWLEDGMENTS
We would like to thank Tracy Frazee for her assistance with the IRB submission, database information retrieval and manuscript preparation.
Contributor Information
Roberta M diFlorio Alexander, Email: roberta.m.diflorio@hitchcock.org.
Steffen J Haider, Email: steffen.haider@gmail.com.
Todd MacKenzie, Email: Todd.A.MacKenzie@Dartmouth.edu.
Martha E Goodrich, Email: Martha.e.goodrich@dartmouth.edu.
Julie Weiss, Email: Julie.Weiss@dartmouth.edu.
Tracy Onega, Email: Tracy.L.Onega@Dartmouth.edu.
REFERENCES
- 1.Kani KK, Moshiri M, Bhargava P, Kolokythas O. Extrahepatic, nonneoplastic, fat-containing lesions of the abdominopelvic cavity: spectrum of lesions, significance, and typical appearance on multidetector computed tomography. Curr Probl Diagn Radiol 2012; 41: 56–72. doi: 10.1067/j.cpradiol.2011.07.005 [DOI] [PubMed] [Google Scholar]
- 2.Montani JP, Carroll JF, Dwyer TM, Antic V, Yang Z, Dulloo AG. Ectopic fat storage in heart, blood vessels and kidneys in the pathogenesis of cardiovascular diseases. Int J Obes Relat Metab Disord 2004; 28(Suppl 4): S58–S65. doi: 10.1038/sj.ijo.0802858 [DOI] [PubMed] [Google Scholar]
- 3.Lim S, Meigs JB. Ectopic fat and cardiometabolic and vascular risk. Int J Cardiol 2013; 169: 166–76. doi: 10.1016/j.ijcard.2013.08.077 [DOI] [PubMed] [Google Scholar]
- 4.McLaughlin T, Lamendola C, Liu A, Abbasi F. Preferential fat deposition in subcutaneous versus visceral depots is associated with insulin sensitivity. J Clin Endocrinol Metab 2011; 96: E1756–E1760. doi: 10.1210/jc.2011-0615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chughtai HL, Morgan TM, Rocco M, Stacey B, Brinkley TE, Ding J, et al. Renal sinus fat and poor blood pressure control in middle-aged and elderly individuals at risk for cardiovascular events. Hypertension 2010; 56: 901–6. doi: 10.1161/HYPERTENSIONAHA.110.157370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rinella ME. Nonalcoholic fatty liver disease: a systematic review. JAMA 2015; 313: 2263–73. doi: 10.1001/jama.2015.5370 [DOI] [PubMed] [Google Scholar]
- 7.Bredella MA, Gill CM, Gerweck AV, Landa MG, Kumar V, Daley SM, et al. Ectopic and serum lipid levels are positively associated with bone marrow fat in obesity. Radiology 2013; 269: 534–41. doi: 10.1148/radiol.13130375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miller JW, King JB, Joseph DA, Richardson LC. Centers for Disease Control and Prevention (CDC). Breast cancer screening among adult women-behavioral risk factor surveillance system, United States, 2010. MMWR Suppl 2012; 61: 46–50. [PubMed] [Google Scholar]
- 9.Altobelli E, Lattanzi A. Breast cancer in European Union: an update of screening programmes as of March 2014 (review). Int J Oncol 2014; 45: 1785–92. doi: 10.3892/ijo.2014.2632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pi-Sunyer X. The medical risks of obesity. Postgrad Med 2009; 121: 21–33. doi: 10.3810/pgm.2009.11.2074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Greene AK, Grant FD, Slavin SA. Lower-extremity lymphedema and elevated body-mass index. N Engl J Med 2012; 366: 2136–7. doi: 10.1056/NEJMc1201684 [DOI] [PubMed] [Google Scholar]
- 12.Geys L, Vranckx C, Lijnen HR, Scroyen I. CD36 deficiency blunts effects of diet on regulatory T cells in murine gonadal adipose tissue and mesenteric lymph nodes. Cell Immunol 2015; 298: 33–6. doi: 10.1016/j.cellimm.2015.08.006 [DOI] [PubMed] [Google Scholar]
- 13.Kim CS, Lee SC, Kim YM, Kim BS, Choi HS, Kawada T, et al. Visceral fat accumulation induced by a high-fat diet causes the atrophy of mesenteric lymph nodes in obese mice. Obesity 2008; 16: 1261–9. doi: 10.1038/oby.2008.55 [DOI] [PubMed] [Google Scholar]
- 14.Stark A, Stahl MS, Kirchner HL, Krum S, Prichard J, Evans J. Body mass index at the time of diagnosis and the risk of advanced stages and poorly differentiated cancers of the breast: findings from a case-series study. Int J Obes 2010; 34: 1381–6. doi: 10.1038/ijo.2010.69 [DOI] [PubMed] [Google Scholar]
- 15.McGowan MM, Eisenberg BL, Lewis LD, Froehlich HM, Wells WA, Eastman A, et al. A proof of principle clinical trial to determine whether conjugated linoleic acid modulates the lipogenic pathway in human breast cancer tissue. Breast Cancer Res Treat 2013; 138: 175–83. doi: 10.1007/s10549-013-2446-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Obeid JP, Stoyanova R, Kwon D, Patel M, Padgett K, Slingerland J, et al. Multiparametric evaluation of preoperative MRI in early stage breast cancer: prognostic impact of peri-tumoral fat. Clin Transl Oncol 2017; 19: 211–8. doi: 10.1007/s12094-016-1526-9 [DOI] [PubMed] [Google Scholar]




