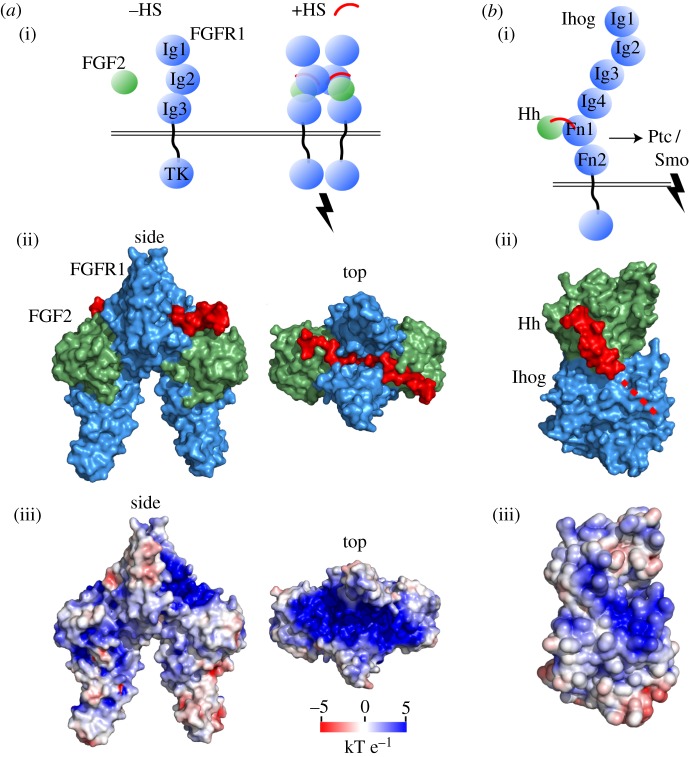
Figure 2.
GAG activators. (a) (i) Secreted FGF (green) interacts weakly with its receptor FGFR (blue). HS (red) stabilizes the interaction and activates the FGFR dimer for downstream signalling through intracellular tyrosine kinase (TK) domains. (ii) The symmetric complex of two human FGF : two human FGFR (Ig2 and Ig3) : two heparan decasaccharide is shown in a molecular surface representation (PDB: 1FQ9). (ii) The protein electrostatic surface potential in the FGF2 : FGFR2 assembly shows a contiguous, positively charged surface compatible for HS binding. (b) (i) Tighter binding of secreted Hedgehog (green) to insect receptor Ihog (blue) with HS allows stronger interaction with the Patched co-receptor, thereby relieving inhibition of Smoothened for pathway activation. (ii) Crystal structure of fly HhN (green) and domains Fn1–Fn2 of fly co-receptor Ihog solved in complex with HS (PDB: 2IBG). HS is crystallographically unresolved and is partly modelled by superposition with ShhN-HS (PDB: 4C4N), followed by the likely continuation of HS along the dotted line to staple the complex. (iii) Electrostatic surface coloured in the same scaling as in (a), with a contiguous positively charged region spanning the Hh–Ihog interface. Electrostatic surface potential was calculated with APBS [11].