Abstract
Zolpidem is widely prescribed for the treatment of insomnia and is used to both induce and maintain sleep. Previously, zolpidem was thought to have low abuse potential; however, several reports have documented dose escalation and abuse in the past two decades. Here, we report the case of a patient with high-dose zolpidem dependence who underwent polysomnography (PSG) and the Multiple Sleep Latency Test (MSLT). The patient, a 29-year-old man, was administered zolpidem at doses of 300 to 1,200 mg/day, but he abused zolpidem to feel energetic. Consequently, he had a car accident while on a high dose, which the PSG revealed caused activation instead of sedation. The MSLT showed excessive daytime sleepiness despite a lack of subjective sleepiness under this condition. Our findings suggest that disrupted sleep and daytime sleepiness caused by supratherapeutic zolpidem doses could place individuals at high risk for accidents, including those who are unaware of sleepiness.
Citation:
Ohshima H, Kotorii N, Takii M, Hiejima H, Habukawa M, Kuwahara H, Uchimura N. Polysomnographic sleep disturbances due to high-dose zolpidem use: a case report. J Clin Sleep Med. 2018;14(11):1949–1952.
Keywords: abused, polysomnography, zolpidem
INTRODUCTION
Zolpidem is a nonbenzodiazepine hypnotic agent that binds selectively to the α1 subunit-containing gamma-aminobutyric acid (GABA)A receptor. It is widely prescribed for the treatment of insomnia, both to induce and maintain sleep. Although it was initially assumed that tolerance is not likely to develop in patients administered the drug and that the drug has minimal abuse potential, there have been several case reports documenting dose escalation and abuse over the past two decades.1,2 This case study aims to describe the objective sleep and daytime arousal levels before and after detoxification for high-dose zolpidem.
REPORT OF CASE
A 29-year-old, single, man of Japanese descent with no prior history of drug abuse or psychiatric illness was first prescribed zolpidem for insomnia at a dose of 10 mg/day at bedtime at the age of 24 years. Within 1 year, he occasionally increased the dose to 40 mg/day based on his own judgment to fall asleep faster, which sometimes caused anterograde amnesia. A few years later, at the age of 28 years, the patient was promoted to the position of store manager; subsequently, his insomnia worsened because of increased work pressure, and hence he visited multiple physicians to obtain prescriptions for higher doses of zolpidem. He started taking zolpidem in the morning as well to be able to sleep during daytime on his day off. Subsequently, he found that administering zolpidem during the daytime made him feel more energetic and sociable than usual, and this response rapidly reinforced his zolpidem abuse. Thereafter, he increased the dose and typically consumed approximately 300 to 400 mg zolpidem per day (50 mg five times in the morning, 30 mg twice midday, and 100 mg at bedtime), which is substantially higher than the prescribed daily dose; occasionally, he even took up to 1,200 mg/day. Other benzodiazepines including quazepam and brotizolam were sometimes prescribed to address his increased difficulty in falling asleep, but they were less effective, and he was unable to wake up until the early afternoon when he self-administered supratherapeutic doses. However, he was able to wake up and resume his activities immediately the next morning without drowsiness even when he had taken a high dose of zolpidem the previous day. When he discontinued zolpidem, he experienced terrible nightmares in addition to cravings and agitation. Under the Japanese health-insurance system that covers about 70% of medical costs of all citizens, he was able to procure numerous zolpidem tablets by consulting physicians at more than 30 hospitals every month with the chief complaint of insomnia. Contrary to his feeling of heightened interest, his performance clearly deteriorated because of drowsiness and restlessness. He started making many superficial errors at work and his subordinates started complaining about him. Frequent anterograde amnesia, which caused loss of such uncomfortable memories, was rather beneficial to him. At 29 years of age, he had a car accident while under the influence of drugs and was dismissed from work. He could not discontinue zolpidem because of the withdrawal symptoms and, therefore, was brought to our hospital for treatment.
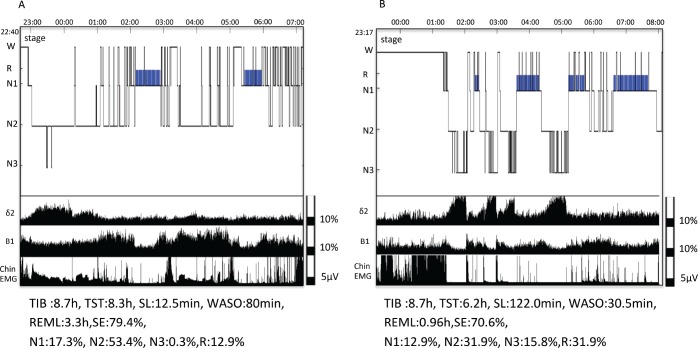
On the day of admission, he had taken 200 mg zolpidem between the morning and mid-afternoon as usual and was hospitalized at 4:00 pm. After the administration of an additional 100 mg before going to bed, he underwent his first polysomnography (PSG) followed by the Multiple Sleep Latency Test (MSLT) on the next day. PSG monitoring revealed that the patient spent more time than expected for his age in stage N1 sleep (64 minutes) and the wake time after sleep onset (WASO) and rapid eye movement (REM) sleep latency were 80 minutes and 180 minutes, respectively (Figure 1). The mean sleep latency in the MSLT was only 5.3 minutes despite the patient having less subjective sleepiness (Epworth Sleepiness Scale [ESS] score = 7).
Figure 1. Polysomnography.
Sleep stage diagrams and time and time-course change in waveform parameters measured during zolpidem abuse (A) and after addiction treatment (B). Under taking zolpidem high dose, elongation of the REM latency, poor slow-wave sleep and frequent nocturnal arousals were observed. N1–N3 = non-rapid eye movement stage 1–3 sleep, PSG = polysomnography, R = rapid eye movement sleep, REML = rapid eye movement sleep latency, SE = sleep efficiency, SL = sleep latency, TIB = time in bed, TST = total sleep time, W = wake, WASO = wake after sleep onset.
Detoxification was started with 10 mg zolpidem, 125 mg chlorpromazine, and 6 mg clonazepam. The zolpidem dose was tapered by 5 mg weekly, and the drug was discontinued after 2 weeks. The clonazepam dose was tapered by 1 mg weekly, and the drug was discontinued after 6 weeks. The chlorpromazine dose was tapered to 50 mg gradually over 12 weeks. Only dysarthria due to excessive tension and irritability were reported as withdrawal symptoms during the first few days after hospitalization. After discontinuing clonazepam, the patient experienced difficulty falling asleep but his subjective sleep quality improved. The second PSG and next-day MSLT with chlorpromazine 50 mg at bedtime were performed 16 weeks after he started the detoxification treatment. The second PSG showed prolonged sleep latency (122 minutes), decreased WASO and stage 1 sleep (30.5 and 51.5 minutes, respectively), and shortened REM latency (58 minutes, Figure 1).
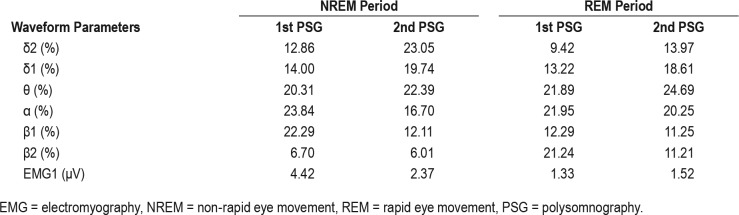
We also measured the time-course changes in wave-form parameters using an automatic sleep analysis system.3 Briefly, electroencephalograms (EEGs, C4-A1) were classified using the interval histogram method (slice line interval, 5 μV) into each of the following wave-form parameters and the individual frequencies were determined: δ2 (0.5–2 Hz), δ1 (2–4 Hz), θ (4–7.5 Hz), α (7.5–13.5 Hz), β1 (13.5–20 Hz), β 2 (20–30 Hz), and γ (30–100 Hz). The mean electromyography (EMG) amplitude for one period was determined. Table 1 shows changes in the EEG and EMG data for the non-rapid eye movement (NREM) and REM periods in the first and second PSG. The lower panel of Figure 1 shows the time-course changes in parameters δ2, β1, and EMG data measured at each PSG.
Table 1.
Waveform parameters.
As shown in Table 1, the proportion of the β1 bands during the NREM period and β2 bands during the REM period was markedly higher in the first PSG than they were in subsequent evaluations. In addition, the mean EMG amplitude during the NREM period was also higher in the first PSG than in other evaluations. The proportion of δ2 bands during the NREM period markedly increased after detoxification. In the follow-up MSLT, the mean sleep latency increased (13.4 minutes) compared to that at baseline (5.3 minutes), whereas the ESS score decreased to 3.
DISCUSSION
In our patient with no prior history of drug abuse, high zolpidem dependence developed despite huge social losses. The neurobiology of drug abuse is generally thought to involve the mesolimbic reward pathway that ultimately might contribute to behavioral dependence through increased levels of dopa-mine and long-lasting synaptic plasticity.4 Tan et al.5 recently reported that benzodiazepines increase dopamine levels similar to addictive drugs (ie, cannabinoids, opioids, and gamma-hydroxybutyric acid) through disinhibition, which relies on α1-containing GABAA receptors in the ventral tegmental area (VTA). The high affinity of zolpidem for GABAA receptors with the α-1 subunit could, to some degree, explain the mechanism underlying its abuse.
From a clinical viewpoint, our patient's zolpidem dependence progressed through ease of awakening and mobility in the morning although he took large doses the previous day. Surprisingly, it was recently reported that therapeutic doses of zolpidem could improve alertness and behavioral responsiveness in patients with severe brain injury.6 Such arousal effects have also been reported with high doses such as 40 mg/day for blepharospasm.7 These paradoxical phenomena could be enhanced at higher doses and may have considerable effects on sleep. Indeed, fragmented sleep accompanied by an increased β1 band, EMG activity, and decreased δ2 band during NREM sleep were observed following the administration of high doses of zolpidem. The underlying mechanism of these PSG findings, which reflect central nervous system arousal, is unknown; however, local perfusion experiments with dopamine compounds have indicated that the VTA plays an important role in the regulation of arousal.8 Dopaminergic axons originating from the VTA also extend to other brain regions, including the limbic structures, dorsal striatum, and prefrontal cortex, which play an important role in stress response and sleep-wakefulness regulation. Interestingly, zolpidem-responsive brain injury cases showed increased cerebral metabolism in these regions.9 Of note, most opioids, which have stimulatory effects on the dopaminergic system, similarly cause increased brief arousals and REM sleep suppression.4 Therefore, it could be speculated that the effects of high-dose zolpidem on the dopaminergic system could autoantagonize its sleep-promoting effect. Furthermore, a reduction in REM sleep and increased REM latency were also observed. The nightmares associated with REM sleep rebound following drug discontinuation, which could also be involved in the physiological dependence, at least in part. The MSLT of the patient showed excessive daytime sleepiness under drug-dependent conditions despite the lack of subjective sleepiness, as indicated by the ESS score. These results indicate that patients might tend to overestimate their wakefulness because of the perceived stimulation.
This case suggests that disrupted sleep and sleepiness without awareness induced by supratherapeutic doses of zolpidem could place patients at high risk for various accidents. To avoid zolpidem abuse, clinicians should be informed about the adverse effects of supratherapeutic doses on the sleep/wake condition and should instruct patients to strictly adhere to the therapeutic dose of zolpidem, which is up to 10 mg/day.
DISCLOSURE STATEMENT
Work for this study was performed at Kurume University School of Medicine, Department of Psychiatry, Fukuoka Japan. All authors have approved the manuscript. The authors report no conflicts of interest.
ABBREVIATIONS
- EEG
electroencephalogram
- EMG
electromyography
- ESS
Epworth Sleepiness Scale
- GABAA
gamma-aminobutyric acid A
- MSLT
Multiple Sleep Latency Test
- NREM
non-rapid eye movement
- PSG
polysomnography
- REM
rapid eye movement
- VTA
ventral tegmental area
- WASO
wake after sleep onset
REFERENCES
- 1.Hajak G, Muller WE, Wittchen HU, Pittrow D, Kirch W. Abuse and dependence potential for the non-benzodiazepine hypnotics zolpidem and zopiclone: a review of case reports and epidemiological data. Addiction. 2003;98(10):1371–1378. doi: 10.1046/j.1360-0443.2003.00491.x. [DOI] [PubMed] [Google Scholar]
- 2.Victorri-Vigneau C, Gerardin M, Rousselet M, Guerlais M, Grall-Bronnec M, Jolliet P. An update on zolpidem abuse and dependence. J Addict Dis. 2014;33(1):15–23. doi: 10.1080/10550887.2014.882725. [DOI] [PubMed] [Google Scholar]
- 3.Kuwahara H, Tanaka M, Mizuki Y, Suetsugi M. An automatic sleep-stage analysis system with off-line high-speed processing using a super minicomputer. Kurume Med J. 1996;43(3):243–248. doi: 10.2739/kurumemedj.43.243. [DOI] [PubMed] [Google Scholar]
- 4.Roehrs T, Roth T. Medication and substance abuse. In: Kryger MH, Roth T, Dement WC, editors. Principles and Practice of Sleep Medicine. 5th ed. Philadelphia, PA: WB Saunders; 2009. pp. 1512–1523. [Google Scholar]
- 5.Tan KR, Brown M, Labouebe G, et al. Neural bases for addictive properties of benzodiazepines. Nature. 2010;463(7282):769–774. doi: 10.1038/nature08758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schiff ND. Recovery of consciousness after brain injury: a mesocircuit hypothesis. Trends Neurosci. 2010;33(1):1–9. doi: 10.1016/j.tins.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Licata SC, Mashhoon Y, MacLean RR, Lukas SE. Modest abuse-related subjective effects of zolpidem in drug-naive volunteers. Behav Pharmacol. 2011;22(2):160–166. doi: 10.1097/FBP.0b013e328343d78a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Honda K, Riehl J, Mignot E, Nishino S. Dopamine D3 agonists into the substantia nigra aggravate cataplexy but do not modify sleep. Neuroreport. 1999;10(17):3717–3724. doi: 10.1097/00001756-199911260-00046. [DOI] [PubMed] [Google Scholar]
- 9.Williams S, Conte MM, Kobylarz EJ, Hersh J, Victor JD, Schiff ND. Quantitative neurophysiologic characterization of a paradoxical response to zolpidem in a severely brain-injured human subject [abstract]. 2009 Neuroscience Meeting Planner; 2009; Chicago, IL. Program No. 541.6/R9. [Google Scholar]