Abstract
Biochemical testing of peritoneal and pleural fluids is carried out widely, although the range of tests likely to be useful is limited in comparison to the repertoire of tests available in a modern biochemistry laboratory. Fluids accumulate when pathological processes cause an imbalance between hydrostatic pressure gradients, capillary membrane permeability and lymphatic capacity, resulting in protein-poor transudates or inflammatory exudates. In peritoneal fluid, albumin is the most useful test, for the calculation of the serum-ascites albumin gradient; protein and LDH have a role regarding risk and diagnosis of spontaneous bacterial peritonitis and amylase may be useful in diagnosing fluid accumulation due to pancreatitis. Peritoneal fluid pH and glucose are not indicated analyses. For pleural fluid, protein and LDH are important in distinguishing between transudate and exudate using Light’s criteria; albumin and the serum-effusion albumin gradient may have a complementary role in patients already on diuretics. Pleural fluid pH is the most useful marker of infection although LDH and glucose are also used. Pleural fluid amylase is often measured but, if raised, is more likely to reflect a malignant process than pancreatic disease as the former is much more prevalent. Tumour markers in both peritoneal and pleural fluids generally have limited diagnostic accuracy for detecting local malignancy. Limited studies validating standard serum test methods for use with pleural and peritoneal fluids have been published but work is progressing in this area both in Australasia and overseas and opportunities exist for contributing to this effort.
Introduction
Biochemical analysis of pleural and peritoneal fluid samples is widely carried out in clinical laboratories. Usually the aim is to diagnose the cause of a patient’s pleural effusion or ascites, although often tests are requested on repeat samples with limited indication for specific analyses. Many tests have been advocated as being useful in specific conditions, but with limited evidence as to their real utility. In addition, many of the testing processes that are used with fluid samples are standard processes designed for use with serum or plasma samples. Fluid samples may or may not resemble plasma in terms of protein and lipid concentrations, and may, at least in principle, be subject to interference because of this matrix difference.1 Thus proper validation and control of methods used for fluid samples have been made a condition of registration by regulatory authorities over the last decade.
This review examines the pathophysiology of peritoneal and pleural fluid formation, the role of biochemical testing in diagnosing the cause of fluid accumulation and the need for, and progress made towards, proper validation of the tests used. In particular, it includes a brief account of recent activities in Australasia working towards clearer guidance on method validation. It does not cover the microbiological and cytological aspects of fluid analysis although these are often vital in the diagnostic pathway.
Formation of Peritoneal and Pleural Fluids in Health and Disease
The pleural and peritoneal spaces normally contain small volumes of fluid that provide lubrication between the parietal and visceral membranes and the organs contained within the space. Formation of these fluids is thought to be governed by the Starling hypothesis. This proposes that net flow into the space is governed by the hydrostatic and plasma colloidal osmotic (oncotic) pressure gradients across the membrane and the permeability to water and proteins of the membranes. Removal of fluid from the spaces is mainly by flow into lymphatics. The walls of the lymphatic ducts contain smooth muscle cells and the ducts themselves have a series of one-way valves. By coordinated contraction and relaxation, lymph is actively pumped away from the peritoneal and pleural spaces, which helps to generate a negative hydrostatic pressure. In addition, the lymphatic ducts are able to increase the flow rate in response to changes in fluid formation rate to ensure that fluid does not accumulate.2,3 In health, these processes are in balance and the volume of fluids remains low and constant. Changes in one or more of these processes can result in accumulation of fluid.
Formation of Ascites
The most common cause of ascites formation is cirrhosis due to chronic liver disease. The pathological process is shown in the Figure. Cirrhosis causes obstruction to blood flow in the hepatic sinusoids, resulting in increased hydrostatic pressure in the portal vein and the release of vasodilators that cause reduced systemic blood pressure and renal perfusion. This prompts activation of the renin-angiotensin-aldosterone and sympathetic nervous systems to cause fluid retention. The increased hydrostatic pressure in the portal circulation causes leakage of low-protein fluid across the capillary walls in the mesenteric and hepatic microcirculation.4 When the rate of this process overwhelms the capacity of the lymphatic system to return the fluid to the circulation, ascites develops.2
Figure.
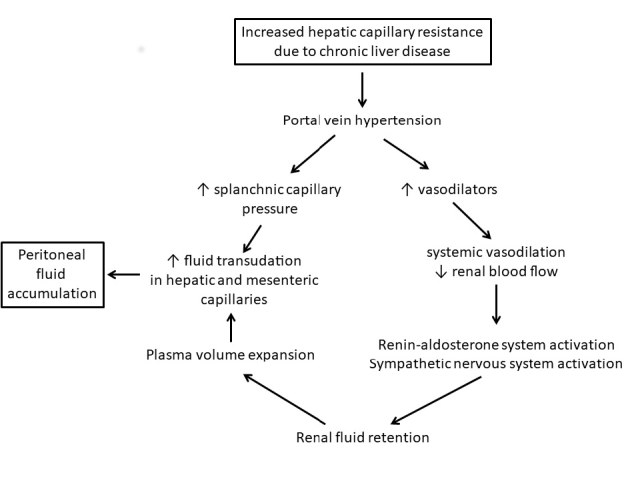
Pathophysiological processes leading to accumulation of peritoneal fluid in chronic liver disease.
Other liver diseases, including hepatic metastatic disease and conditions leading to post-hepatic venous congestion such as heart failure, can cause ascites by a similar process. These processes lead to peritoneal fluid with low albumin and protein concentrations relative to plasma. On the other hand, peritoneal infections and malignancy cause inflammatory processes which lead to the passage of protein-rich fluid into the peritoneal space. Disease of the lymphatic system, by reducing the clearance of peritoneal fluid, may also cause peritoneal effusion.
Ascites formation, along with generalised oedema, is also seen in the low plasma protein states nephrotic syndrome, malnutrition and protein losing enteropathy. Although this is usually explained by the low plasma oncotic pressure in these conditions, at least in nephrotic syndrome, recent studies have indicated that this is not the initial cause of the increased fluid translocation across the capillary wall. Instead the data suggest that renal sodium (Na) conservation, triggered by the increased concentration of proteins in renal tubular fluid, is the main cause of fluid accumulation; oncotic fluid gradients usually do not play a role because the difference between fluid and plasma oncotic pressure tends to remain constant as plasma albumin falls.5
Formation of Pleural Effusions
Pleural fluid occupies the space enclosed by the visceral (inner) and parietal (outer) membranes surrounding the lungs. Formation of the fluid occurs by filtration of plasma across the capillary walls and the pleural mesothelial cells in the parietal membranes, following a hydrostatic pressure gradient.3 Relatively little fluid enters the pleural space across the visceral membrane. Lymphatics opening through the parietal membranes actively drain the pleural spaces, generating the negative hydrostatic pressure in the pleural space.3 In health there is less than 10 mL pleural fluid in each pleural space.
Pleural effusions develop when there is excess hydrostatic pressure in the pulmonary capillaries, when fluid removal is impaired by compromised lymphatic drainage or when protein and cell rich fluid enters the pleural space through leaky capillary and pleural membranes.6 Thus left heart failure, volume overload in critical care and chronic kidney disease patients, and atelectasis (collapsed lung) cause hydrostatic pressure differences across the pleural membranes and result in low-protein concentration pleural transudates. In contrast, lung infection or malignancy provoke inflammatory cells and the pleural mesothelial cells to release cytokines and other mediators, causing increased permeability of the capillary and pleural membranes and allowing the passage of protein- and cell-rich exudate. Impairment of lymphatic function due to fibrin deposition or infiltration by the infection or malignancy will promote the development of an effusion.6 The common causes of exudative pleural effusions include pneumonia, malignancy (especially carcinoma, lymphoma or mesothelioma), tuberculous pleurisy, pulmonary embolism and other inflammatory disorders.
Classification of Pleural and Peritoneal Effusions
Pleural Effusions
Pathological accumulations of pleural fluid are usually classified as transudates or exudates; this classification reflects the likely location of the pathological process causing the effusion. Thus the presence of an exudate usually implies pleural disease, likely to need further investigation, whereas a transudate reflects disease outside the pleural space, and which is often clinically apparent.
Early attempts to distinguish exudates and transudates used specific gravity estimations, although difficulties with accurate measurement and limited specificity were recognised.7 Subsequently direct measurement of protein concentration was applied but both transudates and exudates remained prone to mis-classification.8,9 Measurement of lactate dehydrogenase (LDH) was found to be helpful, particularly in detecting malignant and inflammatory exudates and to improve specificity.10,11 These observations led to the combination of fluid and plasma protein and LDH measurements into a rule which has become known as Light’s criteria.9 The original rule, derived empirically, consisted of: (i) fluid: plasma protein ratio >0.5; (ii) fluid: plasma LDH ratio >0.6; (iii) fluid LDH >200 U/L; if any of these criteria were met the fluid was classified as an exudate.12 Later work revised the fluid LDH activity threshold to 2/3 of the plasma LDH reference interval, to account for differences in results between LDH methods. In the original publication, the sensitivity and specificity for exudative effusion were 99% and 98%, respectively.9 Subsequent authors found similar sensitivity but lower specificity, in the order of 75–80%. Heffner et al.13 carried out a patient-level meta-analysis of studies evaluating Light’s criteria and used receiver operating characteristic (ROC) analysis to determine rational thresholds from the pooled data, with the intent of favouring sensitivity over specificity. This analysis confirmed Light’s thresholds for fluid: plasma protein ratio and fluid:plasma LDH ratio, but showed that the optimal threshold for fluid LDH was 0.45 times the plasma LDH upper reference limit.9 Despite this modification pooled specificity for the modified Light’s criteria was 74.3% although sensitivity was 97.9%. Other combinations of tests, including fluid cholesterol, were examined but it was not clear that any provided superior diagnostic accuracy to Light’s criteria. It was also demonstrated that combinations of tests on pleural fluid alone, i.e. not requiring a simultaneous blood sample, were as effective as Light’s criteria.13 However, despite the obvious practical utility of this approach, it is not widely used. Thus in recent evidence-based guidelines from the British Thoracic Society developed from systematic review of the literature, the diagnosis of pleural fluid exudate or transudate is recommended to be made using Light’s criteria.14 Authorities in Australia recommend these criteria as well and appear to endorse the British guidelines.15 Further evidence of the currency of Light’s criteria, despite their longevity, is provided by a recent review of the diagnosis and management of pleural disease in a leading American journal.16 The currently recommended Light’s criteria are summarised in Table 1.
Table 1.
Criteria for classification of pleural fluids as transudates or exudates. Pleural fluid is classified as an exudate if any of these conditions are fulfilled; otherwise it classified as a transudate.
| Fluid:plasma protein ratio | >0.5 |
| Fluid:plasma LDH ratio | >0.6 |
| Fluid LDH (U/L) | >2/3 × upper reference limit for plasma LDH |
A near simultaneous plasma sample needs to be available to allow the calculation of the ratios but there is little information available as to how contemporaneous the blood and fluid samples should be. In one study in which fluid samples were interpreted with blood collected both simultaneously (within 2 h) and routinely (26–28 h difference), Light’s criteria and serum ascites albumin gradient gave discrepant results in 3 and 5% of cases respectively.17 Thus while plasma samples from a different day will often be satisfactory, a sample collected within 2 h of the fluid is optimal, particularly if the patient’s fluid balance is not in steady state.
A more recent meta-analysis has been carried out, but this has not found any evidence to alter the use of Light’s criteria.18
Classification of Ascites
As for pleural fluids, the traditional classification of ascites into transudate or exudate depending on the protein concentration was frequently found to be misleading. Instead, the serum-ascites albumin gradient (SAAG) is used. This has been shown to correlate well with the difference in hydrostatic pressures between the portal vein and vena cava19 and thus reflects the pathophysiological processes leading to ascites formation outlined above. SAAG values ≥11 g/L are taken to indicate the presence of increased portal vein pressure. In one large study (901 samples) of cases with detailed clinical evaluation the SAAG correctly identified samples for the presence of portal hypertension in 96.7% whereas the fluid protein concentration was correct at predicting exudate or transudate in 55.6% of cases.20 Importantly, SAAG correctly classifies high-portal vein pressure fluids even in the presence of infection or another cause of inflammation.20
Other Tests Helpful in the Diagnosis of Pleural and Peritoneal Effusions
Serum-effusion Albumin Gradient in Pleural Fluid
Light’s criteria classify a proportion of fluids as exudates when they would be expected on clinical grounds to be transudates. This often happens when the patient has been given diuretic therapy before the fluid is collected, which causes extracellular fluid volume contraction and increases the concentration of protein and LDH in the pleural fluid.21 Roth et al.22 first investigated the use of the serum-effusion albumin gradient, by analogy with the use of SAAG in ascites, to improve the specificity of Light’s criteria in this setting and proposed a cut-point of >12 g/L to indicate a transudate. It should be noted that this cut-point was determined empirically from no more than 59 patients and the method of albumin measurement was not stated in the study. Romero-Candeira et al.23 compared Light’s criteria with both serum-to-pleural fluid total protein and albumin gradients and while both gradients were more specific for exudates, especially in those patients who had taken diuretics, the improvement was not statistically significant. Nevertheless, examination of albumin or protein gradient in cases where Light’s criteria have unexpectedly classified the fluid as a transudate has been advocated recently.12
The study by Romero-Candeira et al. employed albumin measurements by immunoturbidimetry, but still employed the serum-effusion albumin gradient threshold of 12 g/L suggested by Roth et al.22,23 The extent to which current routine assay technologies that may differ from those in landmark studies cause systematic errors in the calculation of fluid-serum gradients is not clear. Measurement of albumin in serum by the widely used bromocresol green (BCG) method is well known to suffer positive interference by globulins that is not exhibited by methods based on bromocresol purple (BCP) or immunoturbidimetry, so there is clearly a possibility that application of different methods to fluid samples will require different cut-points for the calculated gradients. Several studies examining the equivalence of albumin results given by different methods on pleural and peritoneal fluids have shown positive bias of 1–3 g/L for BCG based methods compared to immunoturbidimetric methods.24–26 Another study, reported in abstract form, found good agreement between BCG and BCP based methods for peritoneal fluid samples, but significant bias for serum samples, resulting in significantly lower SAAG values when matched serum and fluid samples were measured with the BCP based assay.27 More studies are needed, not only of the effect of different albumin methods on fluid albumin results and gradients but also to clinically validate the cut-points for serum-fluid gradients obtained by different techniques.
LDH
As described above, LDH is measured in pleural fluids as part of the Light’s criteria. A significantly raised fluid LDH is characteristic of parapneumonic effusion or empyema, tuberculous pleuritis or malignancy,10 thus it is not a specific indicator of the cause of an exudative effusion. It is less sensitive and specific than pleural fluid pH as a predictor of impending empyema in parapneumonic effusions, an indicator for early drainage of the effusion.28
In ascites, use of LDH measurement as a marker of peritoneal malignancy was reviewed by Block et al.29 However, likelihood ratios were widely variable between studies and it is not clear that this measurement is useful in this setting. Ascites LDH has also been studied for the diagnosis of secondary vs spontaneous bacterial peritonitis in patients with cirrhotic ascites. The distinction is important as the former condition usually requires early surgical intervention for survival whereas the latter requires medical therapy only. From one small study, the following criteria were developed empirically: (1) ascites protein >10 g/L, (2) glucose <2.8 mmol/L and (3) LDH >225 U/L, with any two results in these ranges being indicative of secondary peritonitis.30 Although sensitive, the authors noted some non-specificity of these criteria. Wu et al. compared these criteria in patients with secondary and spontaneous bacterial peritonitis and sterile ascites and found that the sensitivity and specificity were 97% and 56%, respectively.31 They also examined the possible role of adding CEA and alkaline phosphatase measurements to this algorithm but these have not been incorporated into widespread practice. It should be noted that the method of analysis of LDH was not reported and the sample of patients examined was highly selected so generalisability to usual practice without further study may be limited.
The source of LDH in pleural and peritoneal exudates is probably non-specific, as it is in plasma, with malignant and inflammatory cells as well as damaged tissue and red cells being likely sources in different cases. To the extent that the presence of red cells in the fluid is a reflection of the pathological process in the pleural or peritoneal space LDH results should probably be reported even if the plasma limit for haemolysis index is exceeded; only if the fluid is contaminated with blood due to a traumatic tap would this not apply.
pH
Pleural fluid pH is a useful measure of inflammatory status, so long as the specimen for pH is collected appropriately, as pH rises rapidly on exposure of the fluid to air. Thus, as for blood samples, fluid for pH measurement should be collected directly into a heparinised blood gas syringe and analysed promptly in a blood gas analyser.32 However a recent study found that many laboratories in Australia are not processing pleural fluid samples for pH in this manner.33 Pleural fluid pH in effusions secondary to CHF and cirrhosis is usually >7.4; in these conditions any abnormality in blood pH is reflected in the fluid pH to within pH 0.04.34 Pleural fluid pH results <7.3 are associated with parapneumonic, rheumatoid and tuberculous effusions. Malignancy may give mildly acidic effusions, but pH is rarely <7.3 unless the effusion has been present for some time. In the case of parapneumonic effusions, fluid pH <7.2 is predictive of development of empyema and is an indication for early drainage of the fluid.28,34
Ascites pH, on the other hand, has not been found to be so useful as a marker of peritoneal infection,29 and guidelines advocate that it is not measured.35
Glucose
In uncomplicated pleural and peritoneal effusions, fluid glucose concentration mirrors plasma glucose. In exudates pleural fluid glucose tends to follow pleural fluid pH, with concentrations <3.3 mmol/L seen in empyema, malignant effusions, rheumatoid effusion and often in tuberculous effusion. However glucose is less sensitive than fluid pH in this setting.36 These low glucose concentrations are either due to consumption, or in the case of rheumatoid effusion, impaired transport of glucose across the pleural membranes thickened by the pathological process. Indeed, in rheumatoid effusions the glucose can be particularly low with values <2.2 mmol/L characteristic, especially in long-standing effusions.37 It should be noted that delayed analysis of unpreserved fluid samples can lead to artificially reduced glucose concentrations and some authorities recommend collection into tubes with inhibitors of glycolysis.29,38
Triglycerides
The presence of chylomicrons in pleural and peritoneal effusions gives the fluid a turbid appearance and is characteristic of leakage of chyle from the thoracic duct. The thoracic duct has tributaries starting in the lacteals of the intestinal microvilli, which receive the chylomicrons formed from triglyceride absorption in the small intestinal epithelial cells. Due to coordinated contraction of the thoracic duct chyle flow is upwards. The thoracic duct passes through the peritoneal space and the diaphragm, through the mediastinum, crossing from the right to the left side and past the parietal pleural membrane of the left lung, before emptying into the subclavian vein.39 Congenital malformations or damage to the thoracic duct or its tributaries, either due to surgical or accidental trauma or malignancy can readily therefore allow movement of chylous fluid into the peritoneal or pleural space.39 Thus triglyceride concentrations >1.2 mmol/L in pleural fluid or in ascites are virtually diagnostic of a chylous effusion, but this diagnosis is not excluded with lower triglyceride concentrations if chylomicrons can be demonstrated.40 In that study, lipid electrophoresis was used to demonstrate chylomicrons but this is not widely available in clinical laboratories; observation of a floating lipid layer on allowing the specimen to stand for 24 h may be a practical alternative.
Cholesterol
A number of investigators have examined whether pleural fluid cholesterol adds diagnostic information to Light’s criteria in the classification of exudates and transudates. In the meta-analysis referred to above, fluid cholesterol concentration >1.2 mmol/L had sensitivity and specificity of 89.0% and 81.4%.13
Very rarely, usually after a pleural effusion has been present for a prolonged period, possibly years, the effusion can become enriched in cholesterol. The fluid is turbid and may have a sheen on its surface due to the presence of cholesterol crystals (detectable by polarised light microscopy). Typically the fluid total cholesterol is >5.2 mmol/L and the ratio of cholesterol to triglycerides (molar units) is >2.3. Such fluids are known as pseudochylous or chyliform effusions. They are associated with long-standing inflammatory situations, often due to tuberculosis or rheumatoid disease. It is thought that the cholesterol arises from the membranes of dead inflammatory cells in the pleural space.41
Cholesterol measurement in ascites specimens is not generally recommended.35
Creatinine
The role of creatinine measurement in pleural and peritoneal fluids is to detect the presence of urine, probably from a diseased or damaged ureter. Concentrations greater than the prevailing plasma creatinine are highly suggestive, especially if the fluid is a transudate.
Amylase
Amylase measurement in pleural and peritoneal fluids is often advocated to exclude pancreatic disease as a cause of the effusion. Mechanisms by which pleural fluid can contain amylase from the pancreas are not well established but include fistula formation through the diaphragm, mediastinal pancreatic pseudocyst formation, and leakage due to increased microvascular permeability.42,43 However pancreatic disease is an unusual cause of raised pleural fluid amylase (4.5% in one large study44); malignancy tends to be the commonest cause.44,45 Pleural effusions associated with pancreatic disease demonstrated pancreatic isoamylase while all other causes demonstrated the salivary isoenzyme42 but amylase isoenzyme analysis is rarely carried out in current practice. Higher specificity might be obtained by measurement of fluid lipase but there are only a few case reports published and no systematic studies that these authors could find.46 Because of the non-specificity of pleural fluid total amylase measurement, Branca et al. recommended that it should not be measured routinely; the main indication was cases of recurrent pleural effusion of uncertain cause.45
One situation in which urgent measurement of pleural fluid amylase may be indicated is that of oesophageal rupture, when rapid diagnosis and treatment can be life-saving. Raised fluid amylase results (salivary isoenzyme) are typical in this situation, however available case series are small as the condition is unusual.45 Raised fluid total amylase in this situation will be detected by most clinically used amylase assays as they detect both salivary and pancreatic isoenzymes; isoenzyme-specific amylase assays are not commonly available in current clinical practice.
Increased amylase in peritoneal fluid appears to be a reliable marker of pancreatitis as a cause of ascites. In one small series, patients with ascites but without pancreatitis gave fluid amylase results no more than 1.55 times the serum amylase upper limit of normal whereas the two patients with ascites ascribed to pancreatitis had fluid amylase greater than five times the serum upper limit.47 Again fluid lipase may be useful to increase specificity but larger studies are needed to confirm this observation.48
Adenosine Deaminase
Measurement of adenosine deaminase (ADA) has been advocated to help diagnose tuberculous pleural and peritoneal effusions. Although considered definitive, microbiological tests have limited sensitivity and culture has to be prolonged for several weeks before being reported negative. Thus a biochemical test is potentially useful, although high diagnostic accuracy is necessary. The prevalence of tuberculosis in Australia is less than 6 per 100,000 and the proportion of those cases with only pleural or peritoneal disease is about 8%.49 ADA in tuberculous effusions is thought to arise from activated monocytes and macrophages in the granulomatous lesions. The most commonly used method in the literature incubates adenosine with the patient’s sample and detects the ammonium produced with phenol and hypochlorite in a Berthelot reaction. Other methods, more amenable to automation, have measured either the ammonium, using a glutamate dehydrogenase reaction, or the inosine, after conversion to hypoxanthine and oxidation with xanthine oxidase.50,51 Diagnostic performance of ADA for both peritoneal and pleural tuberculosis has been assessed in multiple studies. A meta-analysis of ADA in tuberculous pleurisy included 63 studies and found mean sensitivity and specificity were 0.92 and 0.90, respectively, although there was wide variation in individual studies, with the lowest sensitivity and specificity being less than 0.5.52 The reference limit in the included studies varied between 30 and 71 U/L for the most commonly used method, although there was no significant effect of reference limit on the diagnostic accuracy in the meta-analysis.52 Similarly good diagnostic performance of ADA in ascites for peritoneal tuberculosis was reported in a meta-analysis of 16 studies. Pooled sensitivity and specificity were 0.93 and 0.96, respectively.53 The reference limits in the included studies were between 30 and 40 U/L for the most commonly used method in this analysis.53 However combination of ADA with other tests, such as interferon-gamma, is required to optimise predictive value in settings with higher prevalence of tuberculosis.54
Pleural Fluid NT-proBNP
Measurement of pleural fluid NT-proBNP has been advocated to assist the diagnosis of pleural effusion due to congestive heart failure (CHF). Various cut-points ranging from 600–4000 ng/L have been advocated and good diagnostic performance and high area under the ROC curve have been demonstrated in some studies. A meta-analysis published in 2010 found summary sensitivity and specificity were both 94%, with an area under the ROC curve of 0.98.55 However in a series of consecutive patients in a medical intensive care unit, sensitivity of pleural fluid NT-proBNP for CHF as a cause of pleural effusion was 89% and specificity only 73%, highlighting impaired specificity in patients with concurrent septic shock and acute kidney injury.56 These authors suggested that while a fluid NT-proBNP below the diagnostic threshold virtually excludes CHF as a cause of the effusion, a result above the threshold should be interpreted with some caution, especially in the presence of conditions known to raise plasma NT-proBNP.56 In addition, studies have shown that pleural fluid and plasma NT-proBNP are highly correlated,55 so pleural fluid NT-proBNP measurement is probably only indicated when there is uncertainty about the cause of the effusion.
Fewer studies have been carried out with pleural fluid BNP, but those that have showed lower sensitivity and specificity for CHF than NT-proBNP, perhaps because of lower stability of BNP in pleural fluid and shorter circulating half-life.55
Tumour Markers in Peritoneal and Pleural Fluid
A number of studies have examined the possible role of tumour markers in detecting malignancy, either primary (mesothelioma) or secondary, in pleural and peritoneal fluids. For pleural fluids, systematic reviews and meta-analyses have been carried out for several tumour markers separately and in combination.57,58 For CEA, CA15-3, CA19-9, CA-125, CYFRA and NSE, pooled sensitivity and specificity ranged from 38–63% and 88–98%, respectively.58 For combinations of CEA with CA125, CA 15-3, CA 19-9 and CYFRA and for CA 15-3 with CYFRA, pooled sensitivity and specificity were 58–89% and 92–98%, respectively.57 For peritoneal fluids a meta-analysis of studies examining CEA as a marker of malignancy associated ascites found a pooled sensitivity and specificity of 43 and 95.5%, respectively.59 A diagnostic accuracy study of CEA, AFP and CA19-9 found moderate sensitivity (16–32%) at cut-points that yielded 95% specificity. Generally then, these studies indicate that pleural and peritoneal fluid tumour markers are not reliably sensitive for detecting the presence of malignancy, although if raised concentrations are found the probability of malignancy is higher.
It should be noted that serum CA125 is almost always increased in patients with peritoneal effusions, due to mesothelial irritation by the presence of fluid.60 It is therefore not helpful in detecting the presence of malignancy.35
Validation of Standard Chemistry Tests for Use with Body Fluids
Regulatory Framework
Measurement of biochemical quantities in pleural and peritoneal fluids have been carried out for decades. Usually the standard protocol for serum or plasma has been used with little published validation of the accuracy of the procedure applied to fluid samples. The major theoretical concern is that differences in the composition of body fluids compared to serum would lead to bias in the measurements due to so-called matrix effects.1 Another concern relates to using the tests at concentration ranges outside the range usually encountered with serum samples.
In recent years, regulatory authorities have expressed concern that such testing has been inadequately validated. In Australia, as in other countries, laboratory accreditation is governed by ISO15189, which specifies that standard test procedures used outside the scope for which they have been validated should be validated by the laboratory before being used routinely (section 5.1.3.3).61 More explicitly, in Australia, the Therapeutic Goods Administration has classified standard tests applied to sample types not specified in the manufacturer’s instructions as ‘In-house In Vitro Diagnostic Devices’ (in-house IVDs).62 Following on from this, the National Pathology Accreditation Advisory Council (NPAAC), which supervises laboratory accreditation by the National Association of Testing Authorities (NATA), has specified the evidence of validation that laboratories need to provide for all their in-house IVDs63 and a guidance document from NATA provides more information on how this can be carried out.64 In summary, with reference to the use of standard tests with pleural and peritoneal body fluids, these documents indicate that when a TGA approved commercially supplied test kit is used for a sample type not specified in the product information document then a complete method validation study must be done, including assessment of both analytical and clinical performance.63 With reference to body fluid analyses, some of these validation studies, particularly regarding clinical performance, are probably beyond the capabilities of most laboratories and may be disproportionate to the clinical risk involved. Similar requirements are put on laboratories in USA under regulations supervised by the Federal Drugs Administration and Clinical Laboratories Improvement Act.
Published Validation Studies
Several studies of analytical performance of serum tests applied to body fluids have been published. Performance of the Ektachem 400 dry-slide analyser with simulated and authentic body fluid samples was examined, and results compared to those from a standard ‘wet’ chemistry system.65 However, several key analytes were not covered in this study including total protein, albumin and LDH. In another study, the performance of the protein and albumin assays applied to peritoneal fluid on the Technicon Chem 1 analyser was compared to a manual biuret procedure and an immunoturbidimetric method, respectively.25 While the total protein assay gave results that agreed well, the albumin assay demonstrated a positive bias compared to the immunoturbidimetric method which the authors ascribed to the non-specificity of the BCG albumin method known to affect serum assays. A validation study of chemistry tests applied to fluids using the Beckman Coulter AU series analysers was carried out by Lin et al.66 While most tests, including albumin, gave close to 100% recovery in spiking and dilution experiments and adequate imprecision, LDH in fluids stored at 4°C was found to be unstable with >30% loss of activity over 7 days. These authors also undertook a small clinical validation study of tests used to distinguish exudates and transudates and demonstrated sensitivity and specificity consistent with those in the literature cited above, which illustrates one approach that laboratories can take in tackling this issue.66 Owen et al. tested the recovery of analytes spiked into body fluid samples using a Cobas 8000 series analyser.67 For peritoneal and pleural fluids, all analytes tested gave recoveries between 90% and 110% and all but rheumatoid factor and bilirubin in pleural fluid gave recoveries within ±5% of 100%. While the analytes tested included total protein, LDH, glucose, amylase, triglycerides and cholesterol, findings for albumin measurement were not included. In a comprehensive validation of chemistry tests applied to fluids on the Roche Cobas 501, spiking and dilution recovery experiments generally performed well, but again, stability of LDH during storage was limited and less than claimed for serum or plasma in the reagent product information document.24 These authors also examined the effect of haemolysis, icterus and lipaemia on fluid test results, which highlighted that limits for these factors for serum analysis in the product information document may not necessarily be appropriate for fluid analyses. They included a description of a streamlined protocol for ‘in-production’ test verification when requests for unusual non-validated test and fluid combinations are received during normal operation.24 Kaleta et al. carried out a detailed assessment of CEA, CA19-9 and AFP in peritoneal fluid using the Beckman Coulter UniCel DxI800 analyser.68 Recovery studies gave >90% in each case, intra- and inter-assay imprecision was acceptable and the effects of haemolysate, bilirubin and fat emulsion on the results were defined. In a conference presentation, Chung and Jones demonstrated an approach to accuracy testing by dilution of fluids into serum, with few samples demonstrating poor accuracy.69 It is probable that more analytical validation studies have been published in abstract form but these may have limited availability. In summary these studies have not shown any major problem with applying tests designed for serum to analysis of body fluids. The major exception is albumin, but this showed a bias that was consistent with the known characteristics of the method used.25
Situation in Australasia
In an effort to determine how close Australian laboratories were to meeting the NPAAC requirements on fluid analysis, the Victorian branch of the Australasian Association of Clinical Biochemists carried out an extensive questionnaire survey of laboratory practice.70 A wealth of information was produced, but in particular, no laboratory reported that either they or the manufacturer had validated their methods for use in pleural or peritoneal fluids. In 2015 the same group carried out a limited external quality assessment exercise with single-sample peritoneal and pleural fluids.26 Samples were distributed to laboratories chosen to reflect the majority of analytical systems in use. A wide range of analytes was included and performance was assessed using allowable limits developed by the Royal College of Pathologists of Australasia (RCPA) Quality Assessment Programme (QAP). There was good agreement between laboratories for a number of tests discussed above, i.e. protein, albumin, glucose, amylase, cholesterol and creatinine, especially after accounting for inter-method biases known from serum assays. In particular, significant bias was seen between albumin methods based on BCG and BCP. However caution was expressed regarding triglycerides and LDH, for which heterogeneity between laboratories was seen, even when they were using the same method. A more limited study of tumour markers (CEA, HCG, CA125 and CA19-9) in peritoneal fluids was included which suggested that routinely available CEA, HCG and CA125 methods may be suitable. For CA19-9, marked heterogeneity was found which only partially reflected the inter-method heterogeneity seen with serum samples in the RCPA QAP, indicating a need for careful validation work for this analyte. While the absence of unexpected bias in this study is reassuring, it was not intended as a validation study and good agreement between laboratories does not prove absence of sample matrix interference affecting all participants. However, this was the first step to provision of an external QAP for fluid analysis, one of the required elements specified by NPAAC.63 As described above, LDH is a crucial component of the Light’s criteria for pleural fluid. For triglycerides, the heterogeneity seemed to be limited to samples with values between 1 and 1.5 mmol/L, but this is the range in which clinical decisions are taken and is therefore of some concern. Thus further investigation of both these tests, with a view to validating performance, would seem to be urgent priorities.
Useful Tests for Pleural and Peritoneal Fluids
Table 2 lists the tests for which the evidence supports clinical utility and which therefore should be available with validated assays in Australasian clinical laboratories. These are the tests to which immediate efforts of validation are likely to be most useful. Table 2 is not an all-inclusive list of tests and their indications and laboratories are likely to receive test requests outside the scope indicated in Table 2. Laboratory staff often balance the conflicting needs of providing a helpful clinical service without providing useless or misleading test results, however further studies are required to guide laboratories on the most effective methods of achieving this with respect to fluids analysis. These issues provide an opportunity for laboratory staff to collaborate with clinical colleagues in respiratory and gastrointestinal medicine to refine the testing and reporting of fluids.
Table 2.
Summary of tests on pleural and peritoneal fluids and their indicated applications.
| Test | Fluid | Diagnostic threshold | Interpretation | Analytical and pre-analytical considerations |
|---|---|---|---|---|
| Protein | Pleural | >0.5 × serum protein | Exudate (Light’s criteria) | Needs simultaneous serum sample |
| LDH | Pleural | Serum:effusion ratio >0.6, LDH>0.67× serum reference limit | Exudate (Light’s criteria); malignancy | Needs simultaneous serum sample. Further study of LDH assay robustness for fluid analysis is indicated |
| Peritoneal | Malignancy | |||
| pH | Pleural | <7.3 | Parapneumonic, tuberculous or rheumatoid effusion | Must be collected and processed anaerobically |
| Peritoneal | Not indicated | |||
| Glucose | Pleural | <3.3 mmol/L | Empyema, rheumatoid, malignant or tuberculous effusion | Preserved specimen or rapid transport to laboratory needed |
| Triglycerides | Pleural, peritoneal | >1.2 mmol/L* | Chylothorax, chyloperitoneum | Further study of triglyceride assay robustness for fluid analysis is indicated |
| Cholesterol | Pleural | >1.2 mmol/L* | May suggest exudate | |
| Peritoneal | >5.2 mmol/L | Pseudochylous (long-standing tuberculous or rheumatoid) effusion | ||
| Albumin | Pleural | Serum effusion albumin gradient >12 g/L* | Suggests transudate | Needs simultaneous serum sample |
| Peritoneal | Serum ascites albumin gradient ≥11 g/L | Portal hypertension (cirrhosis, congestive heart failure) | Needs simultaneous serum sample | |
| Creatinine | Pleural, peritoneal | Effusion creatinine > serum creatinine | Urinothorax, urinoperitoneum, especially if transudate | Need to know prevailing serum creatinine |
| Amylase | Pleural | Effusion amylase > serum upper reference limit* | Usual cause is malignancy; rare – pancreatic fistula or pseudocyst, oesophageal rupture | Only indicated in cases of uncertainty |
| Peritoneal | Ascites amylase >5 × serum URL* | Ascites secondary to pancreatitis | ||
| NT-proBNP | Pleural | 600 – 4000 ng/L | May enhance sensitivity for congestive heart failure | Not needed if plasma NTproBNP or BNP available |
| Tumour markers – CEA, HCG, CA19-9, CA15-3 | Pleural, peritoneal | Limited sensitivity, high specificity (except CA125 – poor specificity) |
Threshold based on limited number of publications.
Conclusions
Biochemical testing of peritoneal and pleural fluids is carried out by most Australasian clinical laboratories. For peritoneal and pleural fluids, the tests for which there is evidence of utility is summarised in Table 2. Notably, the classification of peritoneal fluid samples according to portal vein pressure using the SAAG, first described 35 years ago, is recommended over the transudate-exudate concept.19 On the other hand, the transudate-exudate classification scheme is recommended for pleural fluids using Light’s criteria, unmodified, save for one minor alteration, for over 45 years.9 In both cases, the decision thresholds used were developed by assay methods and analysers no longer in use, but which do not appear to have been reviewed with contemporary analytical systems. In addition, some studies were reported that do not describe the biochemical test methodology,22 making them difficult to interpret in the light of other authors’ work; future studies should include full pre-analytical and analytical details in publications. Most laboratories use test procedures that have been validated for serum and plasma samples but not, specifically, for fluid samples, although a few studies have validated this testing for specific analyser systems. It is now apparent that more widespread validation of these procedures on fluid samples is necessary, a process largely driven by regulatory authorities. Efforts are underway in Australasia and elsewhere to complete the work required to satisfy these requirements.
Footnotes
Competing Interests: None declared.
References
- 1.Clinical and Laboratory Standards Institute. CLSI document C49-A. Wayne, PA, USA: 2007. Analysis of Body Fluids in Clinical Chemistry; Approved Guideline. [Google Scholar]
- 2.Chung C, Iwakiri Y. The lymphatic vascular system in liver diseases: its role in ascites formation. Clin Mol Hepatol. 2013;19:99–104. doi: 10.3350/cmh.2013.19.2.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miserocchi G. Physiology and pathophysiology of pleural fluid turnover. Eur Respir J. 1997;10:219–25. doi: 10.1183/09031936.97.10010219. [DOI] [PubMed] [Google Scholar]
- 4.Moore KP, Aithal GP. Guidelines on the management of ascites in cirrhosis. Gut. 2006;55(Suppl 6):vi1–12. doi: 10.1136/gut.2006.099580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Siddall EC, Radhakrishnan J. The pathophysiology of edema formation in the nephrotic syndrome. Kidney Int. 2012;82:635–42. doi: 10.1038/ki.2012.180. [DOI] [PubMed] [Google Scholar]
- 6.Zocchi L. Physiology and pathophysiology of pleural fluid turnover. Eur Respir J. 2002;20:1545–58. doi: 10.1183/09031936.02.00062102. [DOI] [PubMed] [Google Scholar]
- 7.Paddock FK. The Diagnostic Significance of Serous Fluids in Disease. N Engl J Med. 1940;223:1010–5. [Google Scholar]
- 8.Carr DT, Power MH. Clinical value of measurements of concentration of protein in pleural fluid. N Engl J Med. 1958;259:926–7. doi: 10.1056/NEJM195811062591909. [DOI] [PubMed] [Google Scholar]
- 9.Light RW, Macgregor MI, Luchsinger PC, Ball WC., Jr Pleural effusions: the diagnostic separation of transudates and exudates. Ann Intern Med. 1972;77:507–13. doi: 10.7326/0003-4819-77-4-507. [DOI] [PubMed] [Google Scholar]
- 10.Chandrasekhar AJ, Palatao A, Dubin A, Levine H. Pleural fluid lactic acid dehydrogenase activity and protein content. Value in diagnosis. Arch Intern Med. 1969;123:48–50. [PubMed] [Google Scholar]
- 11.Erickson RJ. Lactic dehydrogenase activity of effusion fluids as an aid to differential diagnosis. JAMA. 1961;176:794–6. doi: 10.1001/jama.1961.63040220001008. [DOI] [PubMed] [Google Scholar]
- 12.Light RW. The Light criteria: the beginning and why they are useful 40 years later. Clin Chest Med. 2013;34:21–6. doi: 10.1016/j.ccm.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 13.Heffner JE, Brown LK, Barbieri CA Primary Study Investigators. Diagnostic value of tests that discriminate between exudative and transudative pleural effusions. Chest. 1997;111:970–80. doi: 10.1378/chest.111.4.970. [DOI] [PubMed] [Google Scholar]
- 14.Hooper C, Lee YCG, Maskell N. Investigation of a unilateral pleural effusion in adults: British Thoracic Society pleural disease guideline 2010. Thorax. 2010;65:ii4–17. doi: 10.1136/thx.2010.136978. [DOI] [PubMed] [Google Scholar]
- 15.Emergency Care Institute - Clinical Resources. Pleural effusion - classification. 2017. [Accessed 26 January 2018]. https://www.aci.health.nsw.gov.au/networks/eci/clinical/clinical-resources/clinical-tools/respiratory/pleural-effusion/pleural-effusion-classification.
- 16.Feller-Kopman D, Light R. Pleural Disease. N Engl J Med. 2018;378:740–51. doi: 10.1056/NEJMra1403503. [DOI] [PubMed] [Google Scholar]
- 17.Jenkinson F, Murphy MJ. Biochemical analysis of pleural and ascitic fluid: effect of sample timing on interpretation of results. Ann Clin Biochem. 2007;44:471–3. doi: 10.1258/000456307781645978. [DOI] [PubMed] [Google Scholar]
- 18.Wilcox ME, Chong CA, Stanbrook MB, Tricco AC, Wong C, Straus SE. Does this patient have an exudative pleural effusion? The Rational Clinical Examination systematic review. JAMA. 2014;311:2422–31. doi: 10.1001/jama.2014.5552. [DOI] [PubMed] [Google Scholar]
- 19.Hoefs JC. Serum protein concentration and portal pressure determine the ascitic fluid protein concentration in patients with chronic liver disease. J Lab Clin Med. 1983;102:260–73. [PubMed] [Google Scholar]
- 20.Runyon BA, Montano AA, Akriviadis EA, Antillon MR, Irving MA, McHutchison JG. The serum-ascites albumin gradient is superior to the exudate-transudate concept in the differential diagnosis of ascites. Ann Intern Med. 1992;117:215–20. doi: 10.7326/0003-4819-117-3-215. [DOI] [PubMed] [Google Scholar]
- 21.Romero-Candeira S, Fernández C, Martín C, Sánchez-Paya J, Hernández L. Influence of diuretics on the concentration of proteins and other components of pleural transudates in patients with heart failure. Am J Med. 2001;110:681–6. doi: 10.1016/s0002-9343(01)00726-4. [DOI] [PubMed] [Google Scholar]
- 22.Roth BJ, O’Meara TF, Cragun WH. The serum-effusion albumin gradient in the evaluation of pleural effusions. Chest. 1990;98:546–9. doi: 10.1378/chest.98.3.546. [DOI] [PubMed] [Google Scholar]
- 23.Romero-Candeira S, Hernández L, Romero-Brufao S, Orts D, Fernández C, Martín C. Is it meaningful to use biochemical parameters to discriminate between transudative and exudative pleural effusions? Chest. 2002;122:1524–9. doi: 10.1378/chest.122.5.1524. [DOI] [PubMed] [Google Scholar]
- 24.Block DR, Ouverson LJ, Wittwer CA, Saenger AK, Baumann NA. An approach to analytical validation and testing of body fluid assays for the automated clinical laboratory. Clin Biochem. 2018;58:44–52. doi: 10.1016/j.clinbiochem.2018.05.002. [DOI] [PubMed] [Google Scholar]
- 25.Engel H, Bac DJ, Brouwer R, Blijenberg BG, Lindemans J. Diagnostic analysis of total protein, albumin, white cell count and differential in ascitic fluid. Eur J Clin Chem Clin Biochem. 1995;33:239–42. [PubMed] [Google Scholar]
- 26.Calleja J. AACB Harmonisation Workshop 2016. Inter-lab Body Fluid Sample Analysis Survey – Analyte Performance Reviews. [Accessed 11 March 2018]. https://www.aacb.asn.au/documents/item/4368.
- 27.Thompson S, Ding L. The bromocresol purple method of albumin measurement significantly underestimates the serum ascites albumin gradient. Pathology. 2018;50:S95. doi: 10.1111/imj.14101. [DOI] [PubMed] [Google Scholar]
- 28.Heffner JE, Brown LK, Barbieri C, DeLeo JM. Pleural fluid chemical analysis in parapneumonic effusions. A meta-analysis. Am J Respir Crit Care Med. 1995;151:1700–8. doi: 10.1164/ajrccm.151.6.7767510. [DOI] [PubMed] [Google Scholar]
- 29.Block DR, Algeciras-Schimnich A. Body fluid analysis: clinical utility and applicability of published studies to guide interpretation of today’s laboratory testing in serous fluids. Crit Rev Clin Lab Sci. 2013;50:107–24. doi: 10.3109/10408363.2013.844679. [DOI] [PubMed] [Google Scholar]
- 30.Runyon BA, Hoefs JC. Ascitic fluid analysis in the differentiation of spontaneous bacterial peritonitis from gastrointestinal tract perforation into ascitic fluid. Hepatology. 1984;4:447–50. doi: 10.1002/hep.1840040316. [DOI] [PubMed] [Google Scholar]
- 31.Wu SS, Lin OS, Chen YY, Hwang KL, Soon MS, Keeffe EB. Ascitic fluid carcinoembryonic antigen and alkaline phosphatase levels for the differentiation of primary from secondary bacterial peritonitis with intestinal perforation. J Hepatol. 2001;34:215–21. doi: 10.1016/s0168-8278(00)00039-8. [DOI] [PubMed] [Google Scholar]
- 32.Cheng DS, Rodriguez RM, Rogers J, Wagster M, Starnes DL, Light RW. Comparison of pleural fluid pH values obtained using blood gas machine, pH meter, and pH indicator strip. Chest. 1998;114:1368–72. doi: 10.1378/chest.114.5.1368. [DOI] [PubMed] [Google Scholar]
- 33.Ng L, Dabscheck E, Hew M. Diagnosis of complicated parapneumonic effusion by pleural pH measurement is jeopardized by inadequate physician knowledge and guideline-discordant laboratory practice. Respir Med. 2017;122:30–2. doi: 10.1016/j.rmed.2016.11.012. [DOI] [PubMed] [Google Scholar]
- 34.Houston MC. Pleural fluid pH: diagnostic, therapeutic, and prognostic value. Am J Surg. 1987;154:333–7. doi: 10.1016/0002-9610(89)90623-5. [DOI] [PubMed] [Google Scholar]
- 35.Runyon BA. Management of Adult Patients with Ascites Due to Cirrhosis: Update 2012. 2012. [Accessed 25 April 2018]. https://www.aasld.org/sites/default/files/guideline_documents/141020_Guideline_Ascites_4UFb_2015.pdf.
- 36.Light RW. Parapneumonic effusions and empyema. Proc Am Thorac Soc. 2006;3:75–80. doi: 10.1513/pats.200510-113JH. [DOI] [PubMed] [Google Scholar]
- 37.Balbir-Gurman A, Yigla M, Nahir AM, Braun-Moscovici Y. Rheumatoid pleural effusion. Semin Arthritis Rheum. 2006;35:368–78. doi: 10.1016/j.semarthrit.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 38.Tarn AC, Lapworth R. Biochemical analysis of ascitic (peritoneal) fluid: what should we measure? Ann Clin Biochem. 2010;47:397–407. doi: 10.1258/acb.2010.010048. [DOI] [PubMed] [Google Scholar]
- 39.Aalami OO, Allen DB, Organ CH., Jr Chylous ascites: a collective review. Surgery. 2000;128:761–78. doi: 10.1067/msy.2000.109502. [DOI] [PubMed] [Google Scholar]
- 40.Staats BA, Ellefson RD, Budahn LL, Dines DE, Prakash UB, Offord K. The lipoprotein profile of chylous and nonchylous pleural effusions. Mayo Clin Proc. 1980;55:700–4. [PubMed] [Google Scholar]
- 41.Huggins JT. Chylothorax and cholesterol pleural effusion. Semin Respir Crit Care Med. 2010;31:743–50. doi: 10.1055/s-0030-1269834. [DOI] [PubMed] [Google Scholar]
- 42.Joseph J, Viney S, Beck P, Strange C, Sahn SA, Basran GS. A prospective study of amylase-rich pleural effusions with special reference to amylase isoenzyme analysis. Chest. 1992;102:1455–9. doi: 10.1378/chest.102.5.1455. [DOI] [PubMed] [Google Scholar]
- 43.Kim EJ, Pamer J, Woods C, Memoli JW. A Case of Recurrent Right-Sided Pleural Effusion Due to a Mediastinal Pancreatic Pseudocyst. Chest. 2013;144:952A. [Google Scholar]
- 44.Villena V, Pérez V, Pozo F, López-Encuentra A, Echave-Sustaeta J, Arenas J, et al. Amylase levels in pleural effusions: a consecutive unselected series of 841 patients. Chest. 2002;121:470–4. doi: 10.1378/chest.121.2.470. [DOI] [PubMed] [Google Scholar]
- 45.Branca P, Rodriguez RM, Rogers JT, Ayo DS, Moyers JP, Light RW. Routine measurement of pleural fluid amylase is not indicated. Arch Intern Med. 2001;161:228–32. doi: 10.1001/archinte.161.2.228. [DOI] [PubMed] [Google Scholar]
- 46.Iglesias JI, Cobb J, Levey J, Rosiello RA. Recurrent left pleural effusion in a 44-year-old woman with a history of alcohol abuse. Chest. 1996;110:547–9. doi: 10.1378/chest.110.2.547. [DOI] [PubMed] [Google Scholar]
- 47.Runyon BA. Amylase levels in ascitic fluid. J Clin Gastroenterol. 1987;9:172–4. doi: 10.1097/00004836-198704000-00012. [DOI] [PubMed] [Google Scholar]
- 48.Sileo AV, Chawla SK, LoPresti PA. Pancreatic ascites: diagnostic importance of ascitic lipase. Am J Dig Dis. 1975;20:1110–4. doi: 10.1007/BF01070753. [DOI] [PubMed] [Google Scholar]
- 49.Toms C, Stapledon R, Waring J, Douglas P National Tuberculosis Advisory Committee, for the Communicable Diseases Network Australia, and the Australian Mycobacterium Reference Laboratory Network. Tuberculosis notifications in Australia, 2012 and 2013. Commun Dis Intell Q Rep. 2015;39:E217–35. [PubMed] [Google Scholar]
- 50.Oosthuizen HM, Ungerer JP, Bissbort SH. Kinetic determination of serum adenosine deaminase. Clin Chem. 1993;39:2182–5. [PubMed] [Google Scholar]
- 51.Slaats EH, Asberg EG, van Keimpema AR, Kruijswijk H. A continuous method for the estimation of adenosine deaminase catalytic concentration in pleural effusions with a Hitachi 705 discrete analyser. J Clin Chem Clin Biochem. 1985;23:677–82. doi: 10.1515/cclm.1985.23.10.677. [DOI] [PubMed] [Google Scholar]
- 52.Liang QL, Shi HZ, Wang K, Qin SM, Qin XJ. Diagnostic accuracy of adenosine deaminase in tuberculous pleurisy: a meta-analysis. Respir Med. 2008;102:744–54. doi: 10.1016/j.rmed.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 53.Shen YC, Wang T, Chen L, Yang T, Wan C, Hu QJ, et al. Diagnostic accuracy of adenosine deaminase for tuberculous peritonitis: a meta-analysis. Arch Med Sci. 2013;9:601–7. doi: 10.5114/aoms.2013.36904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Skouras VS, Kalomenidis I. Pleural fluid tests to diagnose tuberculous pleuritis. Curr Opin Pulm Med. 2016;22:367–77. doi: 10.1097/MCP.0000000000000277. [DOI] [PubMed] [Google Scholar]
- 55.Janda S, Swiston J. Diagnostic accuracy of pleural fluid NT-pro-BNP for pleural effusions of cardiac origin: a systematic review and meta-analysis. BMC Pulm Med. 2010;10:58. doi: 10.1186/1471-2466-10-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yeh JH, Huang CT, Liu CH, Ruan SY, Tsai YJ, Chien YC, et al. HINT Study Group. Cautious application of pleural N-terminal pro-B-type natriuretic peptide in diagnosis of congestive heart failure pleural effusions among critically ill patients. PLoS One. 2014;9:e115301. doi: 10.1371/journal.pone.0115301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yang Y, Liu YL, Shi HZ. Diagnostic Accuracy of Combinations of Tumor Markers for Malignant Pleural Effusion: An Updated Meta-Analysis. Respiration. 2017;94:62–9. doi: 10.1159/000468545. [DOI] [PubMed] [Google Scholar]
- 58.Nguyen AH, Miller EJ, Wichman CS, Berim IG, Agrawal DK. Diagnostic value of tumor antigens in malignant pleural effusion: a meta-analysis. Transl Res. 2015;166:432–9. doi: 10.1016/j.trsl.2015.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ahadi M, Tehranian S, Memar B, Vossoughinia H, Salari M, Eskandari E, et al. Diagnostic value of carcinoembryonic antigen in malignancy-related ascites: systematic review and meta-analysis. Acta Gastroenterol Belg. 2014;77:418–24. [PubMed] [Google Scholar]
- 60.Zuckerman E, Lanir A, Sabo E, Rosenvald-Zuckerman T, Matter I, Yeshurun D, et al. Cancer antigen 125: a sensitive marker of ascites in patients with liver cirrhosis. Am J Gastroenterol. 1999;94:1613–8. doi: 10.1111/j.1572-0241.1999.01152.x. [DOI] [PubMed] [Google Scholar]
- 61.Standards Australia. SA document AS ISO 15189-2013. Sydney, Australia: SA; 2013. Medical laboratories – Requirements for quality and competence. [Google Scholar]
- 62.Therapeutic Goods Administration. Regulatory requirements for in-house IVDs. Canberra, Australia: TGA; 2016. [Google Scholar]
- 63.National Pathology Accreditation Advisory Council. Requirements for the development and use of in-house in vitro diagnostic medical devices (IVDs) 3rd ed. Canberra, Australia: NPAAC; 2014. [Google Scholar]
- 64.National Association of Testing Authorities. General Accreditation Guidance - Validation and verification of quantitative and qualitative test methods. Rhodes, NSW, Australia: NATA; 2018. [Google Scholar]
- 65.Georgewill DA, Graham GA, Schoen I. Applicability of the Ektachem 400 analyzer for assaying analytes in miscellaneous body fluids. Clin Chem. 1988;34:2534–9. [PubMed] [Google Scholar]
- 66.Lin MJ, Hoke C, Dlott R, Lorey TS, Greene DN. Performance specifications of common chemistry analytes on the AU series of chemistry analyzers for miscellaneous body fluids. Clin Chim Acta. 2013;426:121–6. doi: 10.1016/j.cca.2013.08.011. [DOI] [PubMed] [Google Scholar]
- 67.Owen WE, Thatcher ML, Crabtree KJ, Greer RW, Strathmann FG, Straseski JA, et al. Body fluid matrix evaluation on a Roche cobas 8000 system. Clin Biochem. 2015;48:911–4. doi: 10.1016/j.clinbiochem.2015.05.012. [DOI] [PubMed] [Google Scholar]
- 68.Kaleta EJ, Tolan NV, Ness KA, O’Kane D, Algeciras-Schimnich A. CEA, AFP and CA 19-9 analysis in peritoneal fluid to differentiate causes of ascites formation. Clin Biochem. 2013;46:814–8. doi: 10.1016/j.clinbiochem.2013.02.010. [DOI] [PubMed] [Google Scholar]
- 69.Chung J, Jones GRD. Validation of Biochemical Testing of Fluid Samples by Dilution into a Serum Matrix: a Preliminary Investigation. Clin Biochem Rev. 2015;36:S28–9. [Google Scholar]
- 70.Calleja J, Robinson B, Bittar I, Reidy Y, Doery J, Choy KW, et al. Report on the Findings of the AACB 2014 Body Fluid Survey. Clin Biochem Rev. 2016;37:177–94. [PMC free article] [PubMed] [Google Scholar]


