Abstract
Background
Preoperative anaemia is associated with increased morbidity in patients undergoing major surgery. Whether erythrocytes are the only bone-marrow-derived cell lineage that associates with increased surgical complications is unknown. This prospective observational trial studied the mobilization of endothelial progenitor cells (EPCs) in response to exercise in association with postoperative complications.
Methods
After IRB approval, 60 subjects undergoing major thoracic surgery were exercised to exhaustion (peak ). Peripheral blood collected before and after peak exercise was quantified for EPC lineages by fluorescence-activated cell sorter analysis. Complication analysis was based on the Clavien–Dindo classification.
Results
Exhaustive exercise increased EPC [CD45−133+34+ cells=150 (0.00–5230) to 220 (0.00–1270) cells μl−1; median change (range)=20 (−4,180–860) cells μl−1; P=0.03] but not mature endothelial cell (EC) subpopulations. Pre-exercise levels [odds ratio (OR)=0.86, 95% confidence interval (CI): 0.37–2.00, P=0.72), change after exercise as a continuous variable (OR=0.95, 95% CI: 0.41–2.22, P=0.91) and a positive response after exercise (change >0 cells μl−1; OR=0.41, 95% CI: 0.13–1.28, P=0.12) were not statistically significantly associated with the incidence of postoperative complications. Post-hoc receiver operating characteristic curve analyses revealed that subjects with a CD45−133+34+ increase ≥60 cells μl−1 in response to exercise suffered fewer postoperative complications [86% sensitivity, 48% specificity and AUC=0.67 (95% CI: 0.52–0.81)].
Conclusions
Preoperative exercise induces EPC into the peripheral circulation. Subjects with a poor EPC response had a pre-existing propensity for postoperative complications. This warrants further research into the role of bone marrow function as a critical component to endothelial repair mechanisms.
Clinical trial registration
IRB 2003-0434 (University of Texas M.D. Anderson Cancer Center, Houston, TX, USA).
Keywords: training, vascular
Editor's key points.
-
•
Mobilization of endothelial progenitor cells (EPCs) by preoperative exercise was studied as a predictor of postoperative complications.
-
•
In 60 subjects undergoing major thoracic surgery, the exercised-induced increase in EPC number correlated with reduced postoperative complications.
-
•
Whether this response is causative or associative is unknown, but it could provide a useful marker for preoperative risk stratification.
A bone marrow-derived response that mobilizes cells into the peripheral circulation [e.g. reticulocytes with blood loss, leucocytes with infection, and endothelial progenitor cells (EPCs) for vascular repair] is crucial to regenerative responses that follow injury.1 This cellular response is less appreciated (compared with the humoral/endocrine/metabolic response) component of the surgical stress response and the ensuing repair phase. Failure of a cellular response can have detrimental consequence(s). Preoperative anaemia is associated with increased morbidity and mortality in patients undergoing major surgery.2 Whether erythrocytes are the only bone marrow-derived lineage that associates with increased postoperative complications is unknown.
The mobilization of bone marrow-derived EPC plays a key role in vascular/endothelial repair. New vessel formation is regulated by paracrine effects via angiogenesis and endothelial colony-forming cells (ECFCs) potentially differentiate into mature endothelial cells (ECs) with vessel-forming ability.3 Poor mobilization of EPCs into the peripheral circulation associates with reduced survival after critical illness, including septic shock;4, 5 however, it is unknown whether such poor mobilization is associated with postoperative complications.
Given that short-term exercise mobilizes EPCs into the peripheral circulation in healthy volunteers6 and that EPC mobilization in response to critical illness has prognostic value,5 we hypothesized that preoperative exhaustive exercise to mobilize EPCs, used as a surrogate stressor to mimic surgical stress, has prognostic potential for patients at risk of postoperative complications and potentially provide a novel therapeutic target for reducing postoperative complications. As such, this prospective observational study evaluated the dynamic bone-marrow responsiveness, characterized by EPC mobilization to a planned preoperative stressor, and whether poor bone marrow response is associated with postoperative complications.
Methods
Study population
After Institutional Review Board (The University of Texas, M.D. Anderson Cancer Center) approval, 60 consecutive adult subjects undergoing major thoracic surgery, including oesophagectomy, or lung resection (wedge resection, lobectomy or pneumonectomy), were enrolled in this prospective observational study. Major thoracic surgery was defined as procedures requiring thoracotomy. Thoracoscopic surgeries were not included taking into account the differences in complication rates between minimally invasive and open surgical procedures. A detailed list of surgery types is presented in Table 1.
Table 1.
Summary table of subject baseline characteristics and exercise capacity. *Ethnic group was self-reported. AT, oxygen consumption at anaerobic threshold; max, maximal oxygen consumption during exercise
| Subject characteristics | All subjects (n=53) |
|
|---|---|---|
| N | % | |
| Age [mean (sd), yr] | 60.3 (10.3) | |
| <50 | 9 | 17 |
| 50–59 | 15 | 28 |
| 60–69 | 22 | 42 |
| 70+ | 7 | 13 |
| Gender | ||
| Male | 34 | 64 |
| Female | 19 | 36 |
| Ethnic group | ||
| Caucasian | 43 | 81 |
| Other | 10 | 19 |
| Height [mean (sd), cm] | 170.5 (10.7) | |
| Weight [mean (sd), kg] | 82.3 (17.6) | |
| ASA physical status | ||
| I–II | 3 | 6 |
| III–IV | 50 | 94 |
| Modified Lee Cardiac Risk Index | ||
| 2 | 43 | 81 |
| 3 | 8 | 15 |
| 4 | 2 | 4 |
| Previous neoadjuvant therapy | ||
| None | 18 | 34 |
| Radiotherapy | 3 | 6 |
| Chemotherapy | 8 | 15 |
| Chemoradiation | 24 | 45 |
| Smoking | ||
| Never | 15 | 28 |
| Current/former | 38 | 72 |
| Coronary artery disease | ||
| Yes | 29 | 55 |
| No | 24 | 45 |
| Diabetes mellitus | ||
| Yes | 8 | 15 |
| No | 45 | 85 |
| Hyperlipidaemia | ||
| Yes | 9 | 17 |
| No | 44 | 83 |
| Exercise capacity | ||
| AT (ml min kg−1) | ||
| Mean (sd) | 9.8 (2.7) | |
| Median (range) | 10.0 (5.0–17.4) | |
| Peak (ml min kg−1) | ||
| Mean (sd) | 17.8 (4.2) | |
| Median (range) | 18.4 (9.1–28.5) | |
| Surgery type | ||
| Oesophagectomy | 26 | 49 |
| Pulmonary wedge resection (thoracotomy) | 1 | 2 |
| Pulmonary lobectomy (thoracotomy) | 22 | 42 |
| Pneumonectomy | 4 | 8 |
| One-lung ventilation | 53 | 100 |
Each subject gave written informed consent after receiving a thorough explanation of the study design and protocol. Predefined exclusion criteria included: inability to exercise above their anaerobic threshold (AT), thereby ensuring a valid cardiopulmonary exercise test (CPET) of sufficient exercise load was achieved, and any medical condition that deemed subjects unsatisfactory for surgery after their pre-anaesthetic evaluation, including a recent (<3 months prior) history of myocardial infarction, venous thromboembolism, and cerebrovascular accident.
Preoperative comorbidities were defined as: history of smoking, diabetes mellitus, cardiovascular disease (presence of hypertension, coronary artery disease, peripheral artery disease), history of chemoradiation therapy, modified Lee cardiac risk index >2, and ASA Physical Status Classification score >2 and the Charlson weighted index of comorbidity.
Postoperative complications were defined as: cardiac events, including myocardial ischaemia (with or without myocardial infarction), dysrhythmias, congestive heart failure, and postoperative requirement of vasopressors; pulmonary events, including prolonged intubation, postoperative re-intubation, pneumonia, acute lung injury (ALI), and acute respiratory distress syndrome; wound healing events, including wound infection, empyema, and sepsis; and surgical events, including prolonged air leak (>5 days), oesophageal leak, and any other re-operative event. Detailed definitions of these postoperative complications are displayed in the Supplementary Appendix. Complications were analysed according to the Clavien–Dindo classification.7
A blinded researcher reviewed the medical records for occurrence of these predefined perioperative comorbidities and postoperative complications according to the Clavien–Dindo classification. These data were collected for the period of subjects’ hospital stay.
Study design and intervention
Cardiopulmonary exercise testing
Before exercise, baseline observations (heart rate, arterial pressure, pulse oximetry, ECG) and static pulmonary function tests (forced expiratory volume at 1 s, forced vital capacity, maximal voluntary ventilation) were recorded for all subjects. CPET was performed as a multi-stage incremental (‘ramp workload’) study using a cycle ergometer and a metabolic cart with standardized exercise software (Medgraphic Cardio-2CP system, Medical Graphics Corporation, St Paul, MN, USA) for breath-by-breath analysis of gas exchange.
An initial acclimation period consisted of breath-by-breath gas exchange analysis performed in the supine resting position for 5 min. After acclimation the subject pedalled in the upright position at 60 rpm with minimal resistance (unloaded work) for 3 min. After 3 min, loaded work (increasing pedal resistance, watts per minute) followed a standardized ramp protocol to maximal symptom limited exertion that typically lasted 9–12 min. Exercise was terminated by the subject or by the study investigator if symptoms of cardiovascular, pulmonary distress, fatigue, or both were observed. Gas exchange analysis recorded oxygen consumption ( , ml min kg−1) and carbon dioxide production ( , ml min kg−1) at all phases of exercise. AT (ml min kg−1) was defined as peak at the inflection point as determined by the modified V-slope method of plotting carbon dioxide excretion ( ) against oxygen uptake ( ) during increasing exercise intensity, as described by Wassermann.8 The peak was defined as the highest oxygen consumption achieved during the exercise test.
To ensure a comparable amount of perceived exertion, subjects were required to reach the individual AT during the exercise session and encouraged during exercise to achieve true peak . Subjects failing to reach the AT were excluded.
EPC analysis by flow cytometry
Blood was collected before and 10 min after peak exercise, using EDTA as an anticoagulant. Blood samples were frozen according to the freezing/thawing procedure described by Norden-Zfoni and colleagues.9 In brief, blood was collected in cell processing tubes (Becton Dickinson, Franklin Lakes, NJ, USA) containing sodium citrate and Ficoll and centrifuged at room temperature for 25 min at 1600g within 2 h of collection. The mononuclear cells were transferred into a cryotube and an equal volume of freezing medium (RPMI 1640 with 20% DMSO for a final 10% concentration) added to the cell suspension. Samples then underwent a controlled freeze using an isopropanol bath in a −80°C freezer and then stored in liquid nitrogen until batch analysis.
For analysis, thawing was achieved by washing cells using the same storage medium without DMSO, and samples were enumerated within 60 min of thawing. Circulating EPCs and mature ECs were evaluated by six-colour flow cytometry (Fig. 1) using a panel of monoclonal antibodies including anti-CD45 (to exclude non-EPCs), anti-CD133 (an EPC marker), anti-CD31, CD34, and CD146 (mature EC markers). Appropriate analysis gates were used to enumerate viable and apoptotic EPCs. The combination of Syto16 and 7-AAD was used to gain insight into EC viability10 Necrotic cells were identified as Syto16low/7-AAD+, apoptotic cells as Syto16low/7-AAD−, and viable cells as Syto16bright/7-AAD−.
Fig 1.
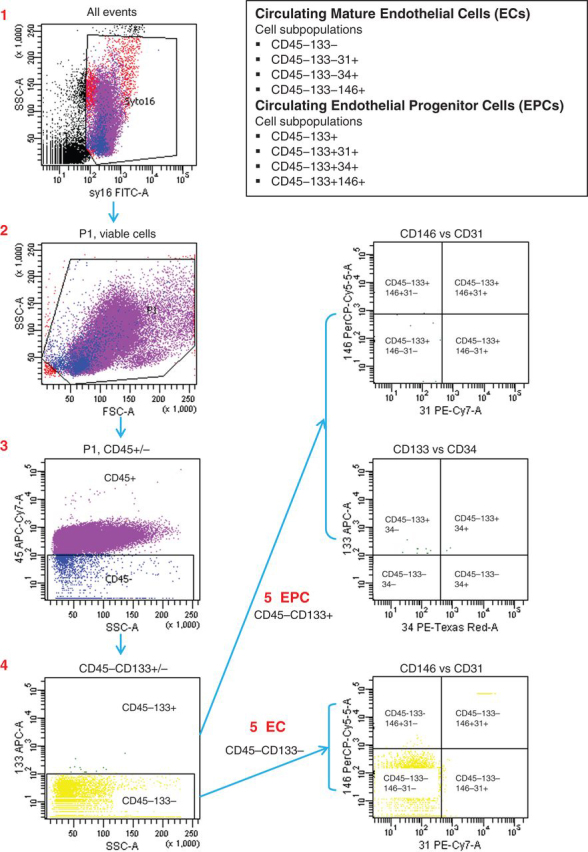
Schematic representation of the strategy used for the quantification of circulating EPCs and ECs by six-colour flow cytometry.
FACSCanto (Becton Dickinson) was used to evaluate cell suspensions after red cell lysis. After acquisition of at least 1×106 cells per blood sample, analysis was considered informative when adequate numbers of cells (>100, typically 300–400) were collected in the enumeration gates. ECs were defined as DNA (Syto16) positive, negative for the haematopoietic marker CD45, positive for EC markers CD31, CD34, and CD146 and negative for the EPC marker CD133. EPCs were depicted by the expression of the stem cell marker CD133+. Figure 1 displays a schematic that summarizes the flow cytometry technique.
Statistical analysis
The primary endpoint was defined as the response of EPC (CD45−133+ lineage) levels to peak exhaustive exercise compared with pre-exercise (baseline) levels. Sample size for the EPC analysis was calculated using the short-term effect of exercise on EPC release 10 min after a symptom-limited dynamic exercise test in volunteers.6 Secondary endpoints included: changes with exercise in other EPC subpopulations (CD45−133+31+ and CD34−133+34+) and mature EC subpopulations (CD45−133−146+, CD45−133−31+ and CD45−133−146+31+), the incidence of postoperative complications and the severity of complications classified in three categories (Clavien-Dindo grade 0, grade I–III, grade IV–V) over the duration of the subjects' hospital stay, and the length of hospital stay.
The changes in EPC and mature EC levels to peak-exhaustive exercise from pre-exercise levels were assessed using the non-parametric Wilcoxon signed-rank test. Associations of subject baseline characteristics with CD45−133+34+ levels were investigated using analysis of variance (anova), linear regression or non-parametric methods as appropriate. Binary logistic regression was used to investigate univariate associations of changes in CD45−133+34+ levels to peak-exhaustive exercise with the incidence of postoperative complications; odds ratios (ORs) were calculated with corresponding 95% confidence intervals (95% CIs). Post-hoc receiver operating characteristic (ROC) curve analyses were also performed to determine the optimal cut-point for changes in CD45−133+34+ levels to peak-exhaustive exercise in predicting postoperative complications. Ordinal logistic regression was then used to investigate associations of changes in CD45−133+34+ levels to peak-exhaustive exercise with the Clavien-Dindo severity of postoperative complications. A P-value of <0.05 was considered to indicate statistical significance. Statistical analyses were carried out using R version 2.14.1 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Sixty consecutive eligible subjects undergoing major thoracic surgery were enrolled in this study (Fig. 2). Three subjects were excluded from data analysis because the procedure was declined for surgical reasons. An additional four subjects were excluded from EPC analysis because they did not have a blood draw because of subject refusal or unavailability of laboratory personnel to process the samples. Subject baseline characteristics are summarized in Table 1.
Fig 2.
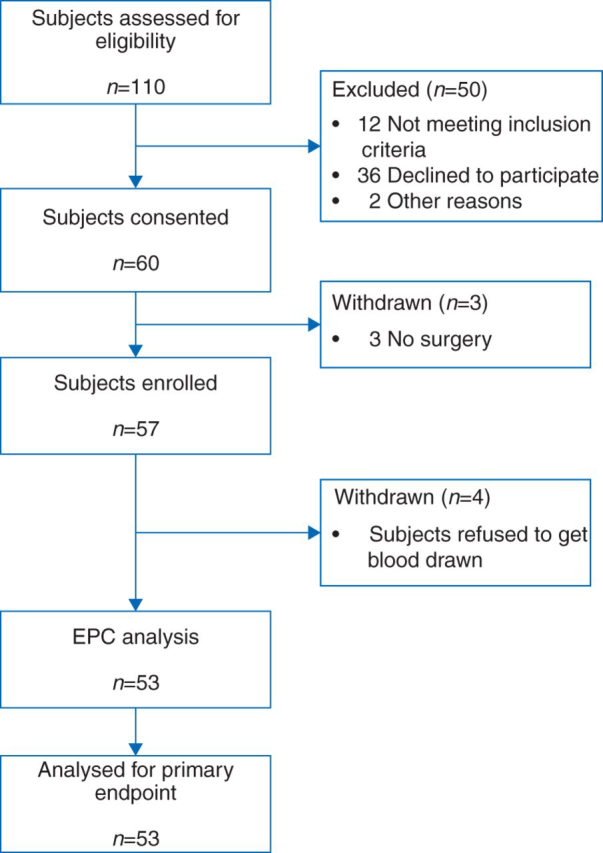
Study flow chart showing subject eligiblity and data analysis.
Circulating EPC and EC levels in response to exhaustive exercise
Compared with baseline levels, exhaustive exercise to peak significantly increased circulating levels of EPC subpopulations [CD45−133+34+ cells: pre-exercise (median [range]) 150 [0.00–5230] cells μl−1 to post-exercise 220 [0.00–1270] cells μl−1; median change [range] 20 [−4180 to 860] cells μl−1; P=0.03] but not that of mature EC subpopulations (Table 2). No statistically significant associations between subject baseline characteristics and change in CD45−133+34+ cell number in response to exhaustive exercise were identified (Table 3).
Table 2.
Effect of preoperative peak exhaustive exercise on circulating levels of EPC and mature EC subpopulations. Data are presented as median and range. Reported P-values for the respective parameters based on Wilcoxon signed-rank test comparing change in postoperative levels compared with preoperative levels
| n | Pre-exercise |
Post-exercise |
Change (post-pre) |
P-value | |
|---|---|---|---|---|---|
| Median (range) | Median (range) | Median (range) | |||
| EPC variables (cells µl−1) | |||||
| CD45−133+ | 53 | 300 (20 to 6800) | 380 (40 to 10 200) | 20 (−6430 to 9580) | 0.07 |
| CD45−133+31+ | 53 | 100 (0 to 4440) | 140 (0 to 2150) | 20 (−3390 to 1140) | 0.06 |
| CD45−133+34+ | 53 | 150 (0 to 5230) | 220 (0 to 1270) | 20 (−4180 to 860) | 0.03 |
| EC variables (cells µl−1) | |||||
| CD45−133−146+ | 53 | 100 (0 to 3560) | 110 (0 to 3510) | 10 (−2200 to 3340) | 0.26 |
| CD45−133−31+ | 53 | 2010 (120 to 32 300) | 2530 (200 to 48 800) | 110 (−20 600 to 48 100) | 0.33 |
| CD45−133−146+31+ | 53 | 30 (0 to 2190) | 20 (0 to 2220) | 0 (−1310 to 300) | 0.37 |
Table 3.
Univariate associations of subject baseline characteristics and change in circulating EPC (CD45−133+34+) levels in response to exhaustive exercise. BART, brachial artery reactivity testing; FMD, flow-mediated dilation; PSV, peak systolic velocity; x+1 identifies the calculation of change in CD45−133+34+ per unit change in BART variable (FMD%, PSV%)
| Reference | Comparator | Mean change in CD45−133+34+ levels after exercise (cells µl−1) | 95% CI | P-value | |
|---|---|---|---|---|---|
| Subject baseline characteristics | |||||
| Age | ≤60 yr | >60 yr | 226 | (−127, 579) | 0.21 |
| Gender | Female | Male | 173 | (−197, 543) | 0.35 |
| ASA | ≤2 | >2 | 23 | (−751, 798) | 0.95 |
| Lee risk index | ≤2 | >2 | −208 | (−662, 246) | 0.36 |
| Treatment | None | Chemoradiation | 156 | (−255, 566) | 0.76 |
| None | Chemotherapy | 173 | (−386, 732) | ||
| None | Radiotherapy | 372 | (−448, 1193) | ||
| Smoking | Never | Former/current | 302 | (−86, 691) | 0.12 |
| Cardiac disease | No | Yes | −124 | (−482, 234) | 0.49 |
| Diabetes | No | Yes | −418 | (−904, 69) | 0.09 |
| Exercise capacity and BART variables | |||||
| AT | x | x+1 | 63 | (3, 128) | 0.06 |
| Peak | x | x+1 | 38 | (−104, 180) | 0.60 |
| FMD% | x | x+1 | −15 | (−116, 85) | 0.76 |
| PSV% | x | x+1 | 2 | (−11, 14) | 0.79 |
Incidence of postoperative complications
Univariate associations of changes in CD45−133+34+ cell number in response to exhaustive exercise and the incidence of postoperative complications according to the Clavien–Dindo classification are summarized in Tables Table 4, Table 5. Pre-exercise levels (OR=0.86, 95% CI: 0.37–2.00, P=0.72), change after exercise as a continuous variable (OR=0.95, 95% CI: 0.41–2.22, P=0.91) and a positive response after exercise (change >0; OR=0.41, 95% CI: 0.13–1.28, P=0.12) were all not statistically significantly associated with the incidence of postoperative complications.
Table 4.
Summary of postoperative outcomes
| Postoperative outcomes | All subjects (N=53) |
|
|---|---|---|
| n | % | |
| Major complications | ||
| Yes | 22 | 42 |
| No | 31 | 58 |
| Cardiac complications | ||
| Yes | 11 | 21 |
| No | 42 | 79 |
| Pulmonary complications | ||
| Yes | 11 | 21 |
| No | 42 | 79 |
| Surgical complications | ||
| Yes | 10 | 19 |
| No | 43 | 81 |
| Clavien complication worst grade | ||
| Grade 0 | 31 | 58 |
| Grade I | 1 | 2 |
| Grade II | 4 | 8 |
| Grade IIIa | 1 | 2 |
| Grade IIIb | 4 | 8 |
| Grade Iva | 5 | 9 |
| Grade IVb | 7 | 13 |
| Grade V | 0 | 0 |
| Length of hospital stay (day) | ||
| Mean (sd) | 10.5 (12.9) | |
| Median (range) | 8 (2–77) | |
| 0–5 days | 18 | 34 |
| 6–10 days | 19 | 36 |
| 11–20 days | 13 | 25 |
| >20 days | 3 | 6 |
| Length of ICU stay (days) | ||
| Mean (sd) | 2.6 (9.9) | |
| Median (range) | 0 (0–60) | |
| 0 days | 40 | 75 |
| 1–5 days | 8 | 15 |
| 6–10 days | 3 | 6 |
| >10 days | 2 | 4 |
Table 5.
Univariate associations of change in CD45−133+34+ in response to exhaustive exercise and the incidence of postoperative complications. *Odds ratio associated with an increase in 1000 cells µl−1.
| Postoperative complication |
OR | 95% CI | P-value | ||||
|---|---|---|---|---|---|---|---|
| Clavien grade 0 |
Clavien grade I–V |
||||||
| n | % | n | % | ||||
| Pre-exercise CD45−133+34+ levels | |||||||
| As a continuous variable | 0.86* | (0.37, 2.00) | 0.72 | ||||
| Change in CD45−133+34+ after exercise | |||||||
| As a continuous variable | 0.95* | (0.41, 2.22) | 0.91 | ||||
| Change ≤0 (– response) | 9 | 45 | 11 | 55 | |||
| Change >0 (+response) | 22 | 67 | 11 | 33 | 0.41 | (0.13, 1.28) | 0.12 |
Post-hoc ROC curve analysis identified an optimal cut-off-point of 60 cells μl−1 for CD45−133+34+ mobilization in response to exercise to predict postoperative complications, achieving 86% sensitivity, 48% specificity and AUC=0.67 (95% CI: 0.52–0.81). Subjects who exhibited a change in CD45−133+34+ of at least 60 cells μl−1 with exercise suffered fewer postoperative complications (17% vs 54%, OR=0.17, 95% CI: 0.04–0.69). These subjects also had a shorter length of hospital stay [median=6, range (2–21) days compared with median=9, range (2–77) days]; however, this did not reach statistical significance (P=0.08).
Discussion
The primary analysis of this study found that acute preoperative exhaustive exercise, as a ‘physiological stressor’, induces an increase in EPC but not mature EC lineages. This bone marrow-derived mobilization likely reflects the regenerative capacity of the subject. As such, secondary analyses found a ‘dose-response’ effect, with fewer postoperative complications according to the Clavien–Dindo classification in subjects exhibiting an increase in circulating levels of EPC after exercise (responders).
Our hypothesis is that the surgical stress response is phasic, with both humoral and cellular components, and that all phases are integral to an optimal surgical outcome. Phasic components include: early fight/flight phase—epinephrine, cortisol, mobilization of erythrocytes, and leucocytes and their precursors for oxygen transport and immune functions; intermediate procoagulant phase; and a late profibrinolytic ‘reperfusion’ and repair phase—with mobilization of various cell lineages to restore anaemia and tissue injury (including EPC cell lineages).
Endothelial dysfunction is recognized as a risk predictor for adverse cardiovascular events,11, 12, 13, 14 adverse postoperative events,15 and increasingly implicated in the pathogenesis of sepsis16, 17 and ALI.18, 19 Subjects with impaired EPC mobilization are more likely to have underlying endothelial dysfunction. Evidence that endothelial dysfunction and bone marrow responsiveness contribute to adverse outcome (and the converse, that endothelial health promotes recovery) is found in both animal models and in humans. In a murine model of LPS-induced ALI bone marrow-derived progenitor cells sequester within the inflammatory site and differentiate into alveolar epithelial and capillary ECs.20 Suppression of progenitor cells by sub-lethal irradiation of the bone marrow impaired recovery resulting in emphysema-like changes. Reconstitution of the bone marrow prevented these changes.20 In humans, critically ill patients with pneumonia,21 ALI22 and sepsis5 respond with increased levels of circulating EPCs. Those subjects exhibiting lowest levels of EPC had persistent pulmonary fibrotic changes despite recovering from pneumonia and also poorer survival after ALI and sepsis.21, 22 These studies support a prognostic value to the magnitude of EPC release in response to a stressor (e.g. surgical trauma, pneumonia or sepsis).
A prospective analysis of subjects undergoing vascular surgery demonstrated that preoperative endothelial dysfunction, as assessed by flow-mediated dilation, also provides independent prognostic information.15 Risk for cardiovascular events within 30 days of surgery was five-fold higher in those subjects with flow-mediated dilation in the lower 2 tertiles (<8%) than in those in the upper tertile (OR 4.9; 95% CI: 1.5–16; P=0.009). Preserved endothelial function had 95% sensitivity and 98% negative predictive value for cardiovascular events. These studies emphasize that the vascular endothelium, a critical sensor-effector organ interfacing between the blood vessel itself and blood-borne elements in all organs, lies central to vascular–haemostatic–inflammatory homeostasis.
Whether endothelial dysfunction and impaired EPC mobilization and that of other cell lineages is an epiphenomena or causative needs further investigation in terms of mechanism, prognostic value, and therapeutic interventions. The ‘dose–response’ relation observed in our data, with decreasing incidence of postoperative complications observed with increasing bone-marrow responsiveness and EPC mobilization, supports causation. Once causation is proved identification of non-responders to an elective ‘physiologic stressor’ such as exercise before surgery might allow for improved preoperative risk stratification and potentially facilitate timely preoperative optimization [e.g. preoperative exercise therapy (‘prehabilitation’), statin therapy to promote EPC mobilization through its pleiotropic effects,23 or cell regenerative therapies] to potentially improve surgical outcomes and reduce healthcare expenditure.
Many factors have been described as having important roles in the mobilization of EPCs.24, 25 Among them are growth factors, such as VEGF, placental growth factor, erythropoietin, and angiopoietin-1, pro-inflammatory cytokines such as GM-CSF and GCSF, chemokines such as stromal cell-derived factor-1, hormones such as oestrogens, and lipid-lowering and anti-diabetic drugs, and also physical activity.26
Exercise6 and tissue insult from surgery27 are known to increase the mobilization of EPCs. Exercise might enhance endothelial function through: shear stress-associated improvement of endothelial function, with increased endothelial nitric oxide synthase (NOS-3) expression and phosphorylation;28, 29 attenuation of vascular oxidative stress by higher local extracellular superoxide dismutase activity;30 and recruitment of bone marrow-derived circulating progenitor cells to the injured endothelial layer to either promote endothelial repair via paracrine mechanisms or differentiate into mature ECs.31 The short-term effect of exercise on the release of EPC has been reported in volunteers performing exhaustive dynamic exercise with blood samples obtained 5–10 min after symptom-limited exercise testing.6 Further, studies have reported a significant increase in circulating EPC levels after exercise training, ranging in duration from 7 days to 1 yr.32, 33, 34, 35 Importantly, this stimulatory effect of exercise on EPC mobilization has been reported not only in trained athletes36 and healthy subjects,37 but also in subjects with known cardiovascular disease.33 However, the duration and intensity of exercise needed to adequately stimulate EPC is still unclear.38
Laufs and colleagues reported increased circulating EPC levels after training with 30 min of moderate exercise (80–100% velocity of the individuals' AT) but not after short term (10 min) of running in healthy subjects.37 In elderly subjects, with documented coronary artery disease, a 4 week exercise programme increased circulating EPC levels, while a more recent study showed that a shorter (15 days) cardiac rehabilitation programme increased EPC in relation to improved exercise capacity.39 A 3 month cardiac rehabilitation programme reported a two-fold increase in circulating EPC levels and a three-fold increase in colony-forming units, with an increase in blood nitrite concentrations and a reduced EPC apoptosis.40
Our study has several limitations. (i) The study design was chosen to be observational and exploratory in nature; therefore, cautious interpretation of the results is required because of the lack of characteristics of a randomized, controlled trial. (ii) Our data do not allow us to speculate about the possible effect of exercise on clinical improvement of endothelial function. Although we were able to show a quantitative increase in EPCs shortly after exercise, it remains uncertain whether mobilized EPCs are fully functional and able to restore endothelial function. Uncertainty remains, whether these cells are able to travel from the bone marrow to the blood vessels and are immured into the endothelial layer, as part of a homing process. (iii) The investigation period of the EPC analysis was relatively short and limited to the first 10 min after peak exercise. The long-term effects of exercise on EPC release were not tested in our study. (iv) Although all subjects exercised to their peak ability and above their AT, variability in the intensity of exercise being performed might have influenced the results.
In conclusion, a preoperative ‘physiologic stressor’ of short exhaustive exercise resulted in a cellular ‘stress response’ with increased peripheral circulating EPC levels. Subjects with impaired mobilization suffered a greater incidence of postoperative complications according to the Clavien–Dindo classification. To our knowledge, this is the first trial that investigates the preoperative regenerative capacity of surgical subjects. Identification of ‘non-responders’ to exercise or other physiological stressors before the anticipated insult of major surgery could allow for improved preoperative risk stratification and potentially facilitate timely implementation of preoperative optimization strategies to reduce postoperative complications and thus healthcare expenditure.
Authors’ contributions
R.S.: study design/subject recruitment/data collection/data analysis/scientific input/manuscript writing/reviewing. R.E.Z.: cell analysis/data collection/data analysis/scientific input/manuscript writing/reviewing. A.C.: cell analysis/data collection/data analysis/manuscript writing/reviewing. M.C.: data analysis/scientific input/manuscript writing/reviewing. N.R., P.T., J.V.H., R.M.: scientific input/manuscript writing/reviewing. B.R.: study design/data collection/data analysis/scientific input/manuscript writing/reviewing. H-K.W. performed flow cytometry experiments. M.L.: data analysis/scientific input.
Declaration of interest
None declared.
Funding
This study was funded through an International Anesthesia Research Society (IARS) Clinical Scholar Grant awarded to B.R.
Handling editor: H. C. Hemmings
Footnotes
Supplementary material is available at British Journal of Anaesthesia online.
SUPPLEMENTARY MATERIAL
References
- 1.Yoder MC. Is endothelium the origin of endothelial progenitor cells? Arterioscler Thromb Vasc Biol. 2010;30:1094–1103. doi: 10.1161/ATVBAHA.109.191635. [DOI] [PubMed] [Google Scholar]
- 2.Musallam KM, Tamim HM, Richards T. Preoperative anaemia and postoperative outcomes in non-cardiac surgery: a retrospective cohort study. Lancet. 2011;378:1396–1407. doi: 10.1016/S0140-6736(11)61381-0. [DOI] [PubMed] [Google Scholar]
- 3.Yoder MC. Human endothelial progenitor cells. Cold Spring Harb Perspect Med. 2012;2:a006692. doi: 10.1101/cshperspect.a006692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jung B, Nougaret S, Chanques G. The absence of adrenal gland enlargement during septic shock predicts mortality: a computed tomography study of 239 patients. Anesthesiology. 2011;115:334–343. doi: 10.1097/ALN.0b013e318225cfd7. [DOI] [PubMed] [Google Scholar]
- 5.Rafat N, Hanusch C, Brinkkoetter PT. Increased circulating endothelial progenitor cells in septic patients: correlation with survival. Crit Care Med. 2007;35:1677–1684. doi: 10.1097/01.CCM.0000269034.86817.59. [DOI] [PubMed] [Google Scholar]
- 6.Rehman J, Li J, Parvathaneni L. Exercise acutely increases circulating endothelial progenitor cells and monocyte-/macrophage-derived angiogenic cells. J Am Coll Cardiol. 2004;43:2314–2318. doi: 10.1016/j.jacc.2004.02.049. [DOI] [PubMed] [Google Scholar]
- 7.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wasserman K. The anaerobic threshold measurement in exercise testing. Clin Chest Med. 1984;5:77–88. [PubMed] [Google Scholar]
- 9.Norden-Zfoni A, Desai J, Manola J. Blood-based biomarkers of SU11248 activity and clinical outcome in patients with metastatic imatinib-resistant gastrointestinal stromal tumor. Clin Cancer Res. 2007;13:2643–2650. doi: 10.1158/1078-0432.CCR-06-0919. [DOI] [PubMed] [Google Scholar]
- 10.van der Pol MA, Broxterman HJ, Westra G, Ossenkoppele GJ, Schuurhuis GJ. Novel multiparameter flow cytometry assay using Syto16 for the simultaneous detection of early apoptosis and apoptosis-corrected P-glycoprotein function in clinical samples. Cytometry B Clin Cytom. 2003;55:14–21. doi: 10.1002/cyto.b.10024. [DOI] [PubMed] [Google Scholar]
- 11.Suwaidi JA, Hamasaki S, Higano ST, Nishimura RA, Holmes DR, Jr, Lerman A. Long-term follow-up of patients with mild coronary artery disease and endothelial dysfunction. Circulation. 2000;101:948–954. doi: 10.1161/01.cir.101.9.948. [DOI] [PubMed] [Google Scholar]
- 12.Perticone F, Ceravolo R, Pujia A. Prognostic significance of endothelial dysfunction in hypertensive patients. Circulation. 2001;104:191–196. doi: 10.1161/01.cir.104.2.191. [DOI] [PubMed] [Google Scholar]
- 13.Halcox JP, Schenke WH, Zalos G. Prognostic value of coronary vascular endothelial dysfunction. Circulation. 2002;106:653–658. doi: 10.1161/01.cir.0000025404.78001.d8. [DOI] [PubMed] [Google Scholar]
- 14.Hill JM, Zalos G, Halcox JP. Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N Engl J Med. 2003;348:593–600. doi: 10.1056/NEJMoa022287. [DOI] [PubMed] [Google Scholar]
- 15.Gokce N, Keaney JF, Jr, Hunter LM, Watkins MT, Menzoian JO, Vita JA. Risk stratification for postoperative cardiovascular events via noninvasive assessment of endothelial function: a prospective study. Circulation. 2002;105:1567–1572. doi: 10.1161/01.cir.0000012543.55874.47. [DOI] [PubMed] [Google Scholar]
- 16.Bernard GR, Vincent JL, Laterre PF. Efficacy and safety of recombinant human activated protein C for severe sepsis. N Engl J Med. 2001;344:699–709. doi: 10.1056/NEJM200103083441001. [DOI] [PubMed] [Google Scholar]
- 17.Merx MW, Liehn EA, Graf J. Statin treatment after onset of sepsis in a murine model improves survival. Circulation. 2005;112:117–124. doi: 10.1161/CIRCULATIONAHA.104.502195. [DOI] [PubMed] [Google Scholar]
- 18.Williams EA, Evans TW, Goldstraw P. Acute lung injury following lung resection: is one lung anaesthesia to blame? Thorax. 1996;51:114–116. doi: 10.1136/thx.51.2.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Siflinger-Birnboim A, Johnson A. Protein kinase C modulates pulmonary endothelial permeability: a paradigm for acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2003;284:L435–L451. doi: 10.1152/ajplung.00106.2002. [DOI] [PubMed] [Google Scholar]
- 20.Yamada M, Kubo H, Kobayashi S. Bone marrow-derived progenitor cells are important for lung repair after lipopolysaccharide-induced lung injury. J Immunol. 2004;172:1266–1272. doi: 10.4049/jimmunol.172.2.1266. [DOI] [PubMed] [Google Scholar]
- 21.Yamada M, Kubo H, Ishizawa K, Kobayashi S, Shinkawa M, Sasaki H. Increased circulating endothelial progenitor cells in patients with bacterial pneumonia: evidence that bone marrow derived cells contribute to lung repair. Thorax. 2005;60:410–413. doi: 10.1136/thx.2004.034058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Burnham EL, Taylor WR, Quyyumi AA, Rojas M, Brigham KL, Moss M. Increased circulating endothelial progenitor cells are associated with survival in acute lung injury. Am J Respir Crit Care Med. 2005;172:854–860. doi: 10.1164/rccm.200410-1325OC. [DOI] [PubMed] [Google Scholar]
- 23.Le Manach Y, Coriat P, Collard CD, Riedel B. Statin therapy within the perioperative period. Anesthesiology. 2008;108:1141–1146. doi: 10.1097/ALN.0b013e318173ef8e. [DOI] [PubMed] [Google Scholar]
- 24.Aicher A, Zeiher AM, Dimmeler S. Mobilizing endothelial progenitor cells. Hypertension. 2005;45:321–325. doi: 10.1161/01.HYP.0000154789.28695.ea. [DOI] [PubMed] [Google Scholar]
- 25.Asahara T, Masuda H, Takahashi T. Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ Res. 1999;85:221–228. doi: 10.1161/01.res.85.3.221. [DOI] [PubMed] [Google Scholar]
- 26.Cesari F, Sofi F, Gori AM. Physical activity and circulating endothelial progenitor cells: an intervention study. Eur J Clin Invest. 2012;42:927–932. doi: 10.1111/j.1365-2362.2012.02670.x. [DOI] [PubMed] [Google Scholar]
- 27.Condon ET, Wang JH, Redmond HP. Surgical injury induces the mobilization of endothelial progenitor cells. Surgery. 2004;135:657–661. doi: 10.1016/j.surg.2003.10.012. [DOI] [PubMed] [Google Scholar]
- 28.Hambrecht R, Adams V, Erbs S. Regular physical activity improves endothelial function in patients with coronary artery disease by increasing phosphorylation of endothelial nitric oxide synthase. Circulation. 2003;107:3152–3158. doi: 10.1161/01.CIR.0000074229.93804.5C. [DOI] [PubMed] [Google Scholar]
- 29.Hambrecht R, Wolf A, Gielen S. Effect of exercise on coronary endothelial function in patients with coronary artery disease. N Engl J Med. 2000;342:454–460. doi: 10.1056/NEJM200002173420702. [DOI] [PubMed] [Google Scholar]
- 30.Fukai T, Siegfried MR, Ushio-Fukai M, Cheng Y, Kojda G, Harrison DG. Regulation of the vascular extracellular superoxide dismutase by nitric oxide and exercise training. J Clin Invest. 2000;105:1631–1639. doi: 10.1172/JCI9551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Crosby JR, Kaminski WE, Schatteman G. Endothelial cells of hematopoietic origin make a significant contribution to adult blood vessel formation. Circ Res. 2000;87:728–730. doi: 10.1161/01.res.87.9.728. [DOI] [PubMed] [Google Scholar]
- 32.Laufs U, Werner N, Link A. Physical training increases endothelial progenitor cells, inhibits neointima formation, and enhances angiogenesis. Circulation. 2004;109:220–226. doi: 10.1161/01.CIR.0000109141.48980.37. [DOI] [PubMed] [Google Scholar]
- 33.Sandri M, Adams V, Gielen S. Effects of exercise and ischemia on mobilization and functional activation of blood-derived progenitor cells in patients with ischemic syndromes: results of 3 randomized studies. Circulation. 2005;111:3391–3399. doi: 10.1161/CIRCULATIONAHA.104.527135. [DOI] [PubMed] [Google Scholar]
- 34.Walther C, Gaede L, Adams V. Effect of increased exercise in school children on physical fitness and endothelial progenitor cells: a prospective randomized trial. Circulation. 2009;120:2251–2259. doi: 10.1161/CIRCULATIONAHA.109.865808. [DOI] [PubMed] [Google Scholar]
- 35.Steiner S, Niessner A, Ziegler S. Endurance training increases the number of endothelial progenitor cells in patients with cardiovascular risk and coronary artery disease. Atherosclerosis. 2005;181:305–310. doi: 10.1016/j.atherosclerosis.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 36.Redondo S, Hristov M, Gordillo-Moscoso AA, Ruiz E, Weber C, Tejerina T. High-reproducible flow cytometric endothelial progenitor cell determination in human peripheral blood as CD34+/CD144+/CD3- lymphocyte sub-population. J Immunol Methods. 2008;335:21–27. doi: 10.1016/j.jim.2008.02.011. [DOI] [PubMed] [Google Scholar]
- 37.Laufs U, Urhausen A, Werner N. Running exercise of different duration and intensity: effect on endothelial progenitor cells in healthy subjects. Eur J Cardiovasc Prev Rehabil. 2005;12:407–414. doi: 10.1097/01.hjr.0000174823.87269.2e. [DOI] [PubMed] [Google Scholar]
- 38.Moyna NM, Thompson PD. The effect of physical activity on endothelial function in man. Acta Physiol Scand. 2004;180:113–123. doi: 10.1111/j.0001-6772.2003.01253.x. [DOI] [PubMed] [Google Scholar]
- 39.Cesari F, Sofi F, Caporale R. Relationship between exercise capacity, endothelial progenitor cells and cytochemokines in patients undergoing cardiac rehabilitation. Thromb Haemost. 2009;101:521–526. [PubMed] [Google Scholar]
- 40.Paul JD, Powell TM, Thompson M. Endothelial progenitor cell mobilization and increased intravascular nitric oxide in patients undergoing cardiac rehabilitation. J Cardiopulm Rehabil Prev. 2007;27:65–73. doi: 10.1097/01.HCR.0000265031.10145.50. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


