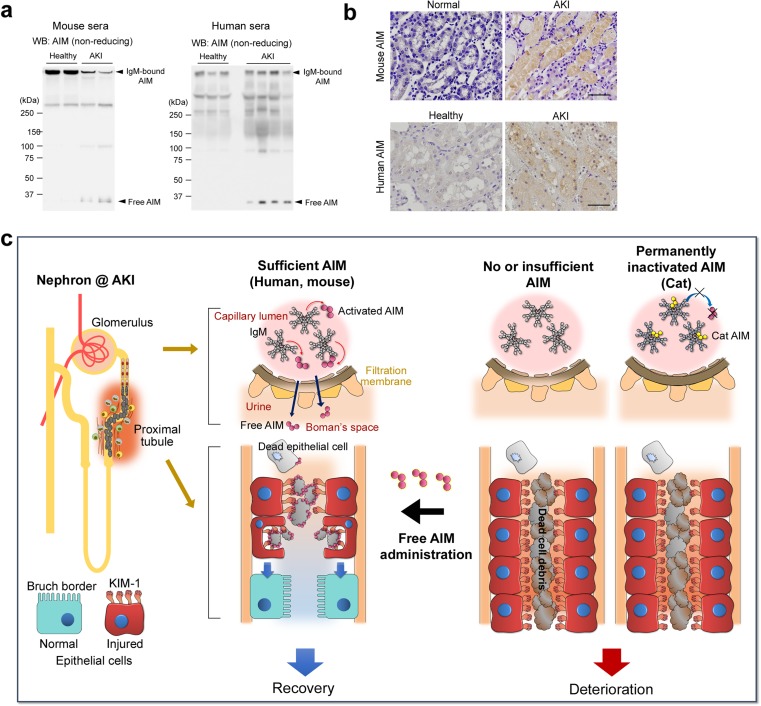
Fig. 5.
Involvement of AIM in AKI pathogenesis. a Western blotting under non-reducing conditions for AIM in sera from control and AKI-induced wild-type mice (n = 2 each, left), and healthy individuals (n = 3) and AKI patients (n = 5). IgM-bound AIM and IgM-free AIM are indicated by arrows. IgM-free AIM is detected only in AKI conditions, whereas IgM-bound AIM is decreased in AKI in mice. b Immunohistochemical staining for AIM of the kidney specimens of healthy and AKI mice (upper panels), as well as a healthy individual and an AKI patient (lower panels). Signals were visualized by horseradish peroxidase/3-diaminobenzidine. Scale bars: 50 μm. Data are modified from a previously published report [13]. c The schematic structure of a nephron and a schema of AIM states and proximal tubules during AKI in wild-type mice, humans, AIM-deficient mice, and cats are depicted. In wild-type mice and most humans, sufficient IgM-free AIM typically dissociates from the IgM pentamer during AKI and is filtrated through glomeruli into the urine, accumulating on the intraluminal dead cell debris and thereby enhancing its clearance by injured proximal tubular epithelial cells (indicated by red cells) via KIM-1, leading to the regeneration of epithelial cells (indicated by blue cells) and AKI recovery. In the absence of AIM (for example, AIM-deficient mice), this debris removal is deficient. In cats, due to the high affinity between AIM and IgM, AIM is unable to dissociate from IgM during AKI, abolishing its excretion in urine; the intraluminal debris therefore cannot be removed efficiently. rAIM administration could be therapeutically applied for AKI treatment