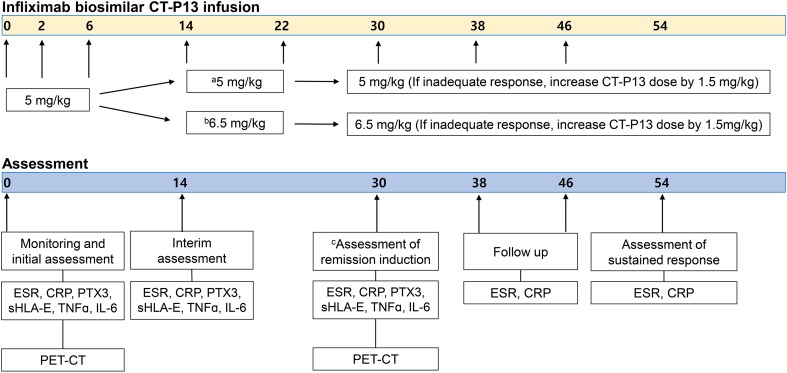
Fig. 1.
Summary of the study design. a Patients whose ESR and CRP improved by ≥ 50% at week 14. b Patients whose ESR and CRP did not improve by ≥ 50% or increased. c Primary end point. ESR erythrocyte sedimentation rate, CRP C-reactive protein, PTX3 pentraxin-3, sHLA-E soluble human leukocyte antigen-E, TNFα tumor necrosis factor-α, IL-6 interleukin-6, ITAS-2010 Indian Takayasu Clinical Activity Score, PET–CT positron emission tomography–computed tomography