Abstract
Aim: Speckle-tracking imaging has been introduced for the precise assessment of vessel mechanics. However, there are no data on the role of this imaging tool in assessing the changes in vasculature with statin therapy, which is known to enhance vascular elasticity.
Methods: This study was a prospective study including 48 statin-naïve patients (age, 58.2 ± 8.4 years; 29.2% male) with hypercholesterolemia. Circumferential carotid artery strain (CAS) and stiffness index (β2) were measured using speckle-tracking imaging before and after 3 months of high-dose pitavastatin treatment (4 mg daily). For the comparison, we measured conventional carotid elasticity parameters and intima–media thickness using B-mode ultrasound at the same time points.
Results: Compared with baseline, there was significant improvement in circumferential CAS (2.98% ± 1.18% to 3.40% ± 1.43%, p = 0.008) and β2 (0.19 ± 0.07 to 0.17 ± 0.08, p = 0.047) after statin therapy. Contrariwise, there were no significant changes in all conventional carotid elasticity metrics and intima–media thickness. When stratifying patients into two subgroups by 10 year atherosclerotic cardiovascular disease (ASCVD) risk, speckle-tracking-derived circumferential CAS and β2 improved significantly only in patients with ASCVD risk ≥ 7.5%.
Conclusions: Short-term treatment with high-dose pitavastatin improved carotid artery elasticity measured by speckle-tracking method, but not conventional parameters by B-mode ultrasound. Speckle-tracking-based measurements may allow the early noninvasive assessment of statin effects on vascular function in hypercholesterolemic patients.
Abbreviations: CAS: carotid artery strain, β1: classic stiffness index, β2: speckle-tracking-derived stiffness index, IMT: intima-media thickness, ASCVD: atherosclerotic cardiovascular disease, CHD: coronary heart disease, LCL-C: low-density lipoprotein cholesterol, HDL-C: high-density lipoprotein cholesterol, CCA: common carotid artery
Keywords: Pitavastatin, Carotid artery elasticity, Carotid artery strain, Speckle-tracking imaging, Hypercholesterolemia
Introduction
Atherosclerosis is a growing problem worldwide, leading to an increased risk of cardiovascular events, including stroke, myocardial infarction, and heart failure1). The loss of arterial elasticity has been suggested as an early marker of atherosclerosis and a strong predictor of subsequent risk of cardiovascular diseases2). Evidence is also emerging that treatment to improve the arterial compliance can potentially reduce cardiovascular morbidity and mortality.
Statins have been the mainstay of therapy for atherosclerotic cardiovascular diseases (ASCVDs), dramatically reducing mortality and morbidity. There are several lines of evidence suggesting that statins can exhibit pleiotropic effects on the cardiovascular system in addition to their cholesterol-lowering properties. Previous studies showed that atorvastatin treatment was associated with better endothelium-dependent vasodilatation3, 4). Other studies also demonstrated that statin therapy improved carotid artery stiffness measured by ultrasound, providing the potential to extend the benefits of statin therapy to asymptomatic subjects with early atherosclerosis5, 6).
However, the impact of statins on carotid artery elasticity has to be further evaluated, because previous results were mainly based on the measurements of changes in carotid diameter obtained by B-mode ultrasound, which is a one-dimensional approach having several limitations in accurately assessing carotid elasticity, such as angle dependency (i.e., increased risk of measuring errors resulted from the deviation of ultrasound beam from the center)7, 8). As part of the efforts to overcome these limitations of conventional techniques, 2-dimensional (2D) speckle-tracking imaging has been introduced as a novel technology allowing the assessment of cardiovascular mechanics with less angle dependency8). As evidence has subsequently mounted for the additional advantages of speckle-tracking imaging over traditional modalities, such as improved sensitivity, this state-of-the-art imaging has been increasingly used not only for early detection of changes in cardiovascular mechanics in various disease conditions9–11) but also for assessment of their improvement after treatments12). Although the application of speckle-tracking imaging has also been extended to the measurement of the mechanical properties of carotid arteries, its utility in evaluating the effect of statin use on carotid elasticity has not been studied to date.
Aim
We sought to determine whether 2D speckle-tracking strain imaging could evaluate the impact of short-term treatment with high-dose pitavastatin on carotid arterial elasticity.
Methods
Study Population and Design
From June 2014 to June 2015, we prospectively screened 66 patients with hypercholesterolemia who were candidates for statin treatment according to the National Cholesterol Education Program: Adult Treatment Panel Ⅲ13). To be eligible, participants were required to meet the following criteria: (1) age from 40 to 80 years, (2) statin-naïve subjects, and (3) lowdensity lipoprotein cholesterol (LDL-C) ≥ 130 mg/dL with more than or equal to two major risk factors for coronary heart disease (CHD) or LDL-C ≥ 160 mg/dL with less than two risk factors. Major risk factors for CHD include (1) ≥ 45 years of age for men or ≥ 55 years of age for women, (2) family history of premature CHD (CHD in male first-degree relative < 55 years or in female first-degree relative < 65 years), (3) current cigarette smoking, (4) blood pressure (BP) ≥ 140/90 mmHg or on anti-hypertensive medication, and (5) high-density lipoprotein cholesterol (HDL-C) < 40 mg/dL. Exclusion criteria were (1) hospitalization for acute coronary syndrome or cerebrovascular disease within the previous 2 months; (2) the use of statin or other lipid-lowering medication within the previous 3 months; (3) impaired hepatic function or a history of liver disease; (4) chronic renal failure; (5) a history of malignancy; (6) any known contraindication to statin therapy, such as statin allergy, ciclosporin use, pregnancy, breastfeeding, myopathy, or lactose intolerance; and (7) failure to obtain informed consent from participants. Of the 66 patients initially screened, 6 patients did not meet eligibility criteria, 11 patients were eligible but refuse to participate, and 1 patient withdrew the consent during follow-up. Finally, a total of 48 patients with hypercholesterolemia were included for final analysis. A flowchart is provided in Fig. 1.
Fig. 1.
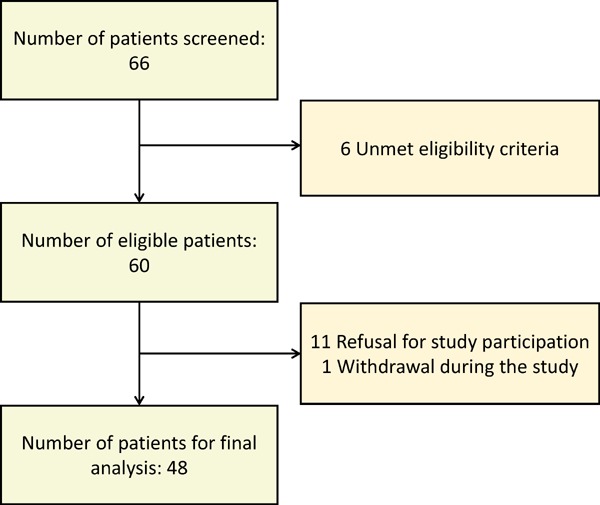
Flowchart of study subjects
On the basis of baseline data collected, an individual's 10 year risk for ASCVD events was calculated using the Pooled Cohort Equations14). Patients were considered to be at elevated risk if an estimated 10 year ASCVD risk was ≥ 7.5%. All subjects received pitavastatin 4 mg daily for 3 months. Study assessments were performed at baseline and 3 months after statin therapy, which consisted of medical history including adverse events, physical examination, BP measurements, carotid ultrasound, and laboratory tests including lipid profiles, liver and kidney function tests, and high-sensitivity C-reactive protein (hs-CRP). The study protocol was approved by our institutional review board and is in accordance with the Declaration of Helsinki. All study subjects provided written informed consent to participate in this study.
Carotid Ultrasound
Ultrasound images were acquired with a GE Vivid E9 system (GE Healthcare) and a 7.5-MHz linear 2D array transducer, following a standardized protocol15). To allow accurate comparison between baseline and follow-up assessments for the same participant, great care was taken into selecting the same site for measurements of carotid ultrasound parameters. Specifically, the distal left common carotid arteries (CCA) were imaged 10 mm inferior to the carotid bulb in transverse and longitudinal sections with electrocardiography gating over at least three cardiac cycles. Furthermore, according to our routine protocol for carotid ultrasound used in clinical practice as well as in clinical trials, sonographers reviewed previous common carotid artery images, particularly focusing on the carotid bulb, obtained during prior examinations on the participant, which enabled to confirm location and orientation of the landmarks of the carotid artery and served as an aid for the sonographers in localizing the carotid bulb. Images were transferred to a workstation equipped with 2D strain software (EchoPAC version 201; GE Healthcare). Then, carotid arterial strain (CAS) analysis was performed using speckle-tracking as previously described7, 16–18). Briefly, all the regions of interest were placed to cover the cross-sectional area of the CCA wall. The software automatically detected frame-to-frame movement of each speckle on the CCA wall during the cardiac cycle. The CCA wall was equally divided into six segments, and each segment was analyzed individually. From each time-strain curve for all six segments, the global circumferential peak systolic strain (%) was determined automatically. Systolic BP (SBP) and diastolic BP (DBP) were measured before and after the carotid ultrasound examination and then averaged. Speckle-tracking-derived stiffness index (β2) was calculated as follows: β2 = ln(SBP/DBP)/strain by speckle-tracking8, 19). Fig. 2 shows a typical example of circumferential CAS measurement.
Fig. 2.
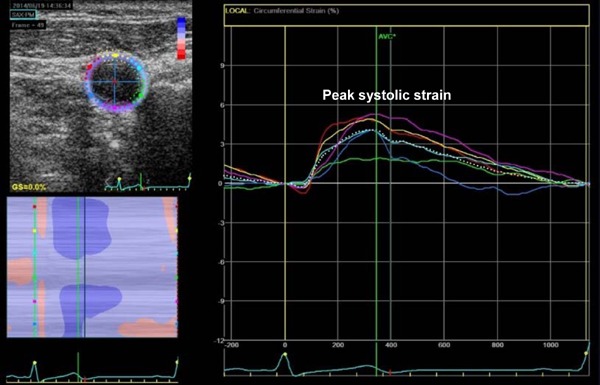
Representative examples of measurements of CAS by speckle-tracking imaging
Conventional carotid artery elasticity metrics, intima–media thickness (IMT), and maximal carotid plaque thickness were also assessed using B-mode ultrasound as previously described5). Briefly, systolic diameter (Ds) and diastolic diameter (Dd) were measured and averaged over three cardiac cycles. Elasticity variables were calculated as follows: strain by B-mode (%) = (Ds−Dd)/Dd, classic stiffness index (β1) = ln(SBP/DBP)/strain by B-mode ultrasound, and distensibility = 1/[ln(SBP/DBP)/strain by B-mode ultrasound × IMT]. Carotid IMT was defined as the distance between the leading edges of the first and second echogenic lines, representing the lumen–intimal interface and the upper layer of the adventitia, respectively. IMT was measured at the far wall of the left CCA, and the mean rather than the maximal IMT was used for the analyses. All analyses were performed by independent observers blinded to the study protocol.
Outcome Measures
The primary outcome was the change in circumferential CAS measured by speckle-tracking imaging after 3 months of pitavastatin treatment. The secondary outcomes were the changes in speckle-tracking imaging-derived stiffness index (β2), conventional carotid elasticity parameters [conventional strain, carotid stiffness (β1), distensibility], mean IMT, and laboratory tests after 3 months of treatment. Analyses were performed in subjects with adherence to statin treatment ≥ 70% and complete data on both baseline and follow-up measurements (per protocol analysis).
Reproducibility Assessment
The interobserver and intraobserver variabilities of speckle-tracking measurements were assessed using intraclass correlation coefficients and Bland–Altman analysis in a random subset of 30 patients. For the assessment of intraobserver variability, measurements were repeated by the same investigator twice, at least 1 week apart. Additional measurements were performed by a second observer blinded to the previous results for the assessment of interobserver variability.
Statistical Analysis
The sample size was determined on the basis of a previous pilot study that adopted similar eligibility criteria as were used in our study5). The continuous variables were expressed as means ± standard deviations or medians (interquartile ranges) as appropriate, whereas categorical variables were expressed as numbers (percentages). Comparisons of continuous and categorical variables were performed using the Mann–Whitney U and Fisher exact tests, respectively. The paired Wilcoxon signed rank test was used to study the change in variables between baseline and 3 months after statin therapy. Two-sided p values < 0.05 were considered statistically significant. All analyses were performed using SPSS version 22.0 (IBM Co., Armonk, NY, USA).
Results
Table 1 shows baseline clinical and laboratory characteristics of study patients. The mean age was 58 ± 8 years, and female predominance was observed (70.8%, [34/48]). Thirty-two patients (66.7%) had hypertension, and 8 patients (16.7%) had diabetes. None of the patients had coronary artery disease. Of the patients, 25 (52.1%) were taking angiotensin-converting enzyme (ACE) inhibitors or angiotensin receptor blockers (ARB), 10 (20.8%) were taking beta-blockers, and 15 (31.3%) were taking calcium-channel blockers (CCB). The medication types and doses were not changed from baseline to the end of pitavastatin treatment. Initial laboratory analysis showed no abnormal findings, except for elevated total cholesterol and LDL-C levels. Among all study participants, 18 patients (37.5%) had a 10 year risk for ASCVD events of ≥ 7.5%. These patients with elevated ASCVD risk were older; more frequently male; more likely to have a history of diabetes mellitus and current smoking; and likely to have lower HDL-C, higher creatinine, and higher hs-CRP levels. Clinical and laboratory characteristics at baseline and at follow-up are summarized in Table 2. Total cholesterol and LDL-C levels significantly reduced after 3 months of pitavastatin treatment, compared with baseline. There were no significant changes in the values of other laboratory values including hs-CRP.
Table 1. Baseline characteristics of the study population.
| Overall | 10-year ASCVD risk | 10-year ASCVD risk | p value | |
|---|---|---|---|---|
| (n = 48) | < 7.5% (n = 30) | ≤ 7.5% (n = 18) | ||
| Clinical data | ||||
| Age (years) | 58.2 ± 8.4 | 54.8 ± 6.9 | 63.8 ± 7.5 | 0.001 |
| Female, n (%) | 34 (70.8) | 27 (90.0) | 7 (38.9) | < 0.001 |
| Body mass index, kg/m2 | 24.8 ± 3.2 | 24.6 ± 3.2 | 25.1 ± 3.2 | 0.694 |
| Body surface area, m2 | 1.66 ± 0.15 | 1.63 ± 0.15 | 1.71 ± 0.13 | 0.020 |
| Systolic blood pressure, mmHg | 123 ± 11 | 121 ± 10 | 126 ± 11 | 0.199 |
| Diastolic blood pressure, mmHg | 74 ± 10 | 74 ± 10 | 74 ± 9 | 1.000 |
| Heart rate, bpm | 70 ± 11 | 69 ± 12 | 72 ± 9 | 0.350 |
| Hypertension, n (%) | 32 (66.7) | 18 (60.0) | 14 (77.8) | 0.343 |
| Diabetes mellitus, n (%) | 8 (16.7) | 1 (3.3) | 7 (38.9) | 0.003 |
| Smoking, n (%) | 8 (16.7) | 1 (3.3) | 7 (38.9) | 0.003 |
| Medication, n (%) | ||||
| ACEI or ARB | 25 (52.1) | 13 (43.3) | 12 (66.7) | 0.145 |
| Beta blocker | 10 (20.8) | 6 (20.0) | 4 (22.2) | 1.000 |
| Calcium channel blocker | 15 (31.3) | 6 (20.0) | 9 (50.0) | 0.052 |
| Laboratory data | ||||
| Total cholesterol, mg/dL | 246.5 ± 19.6 | 248.8 ± 18.0 | 242.6 ± 21.9 | 0.250 |
| LDL-C, mg/dL | 168.8 ± 19.7 | 170.2 ± 15.9 | 166.5 ± 25.1 | 0.456 |
| HDL-C, mg/dL | 56.0 ± 10.6 | 58.2 ± 8.5 | 52.3 ± 12.9 | 0.028 |
| Triglyceride, mg/dL | 144.1 ± 67.3 | 134.9 ± 57.7 | 159.6 ± 80.1 | 0.437 |
| Aspartate Aminotransferase, IU/L | 26.4 ± 13.0 | 26.6 ± 14.1 | 26.0 ± 11.2 | 0.623 |
| Alanine Aminotransferase, IU/L | 29.4 ± 21.9 | 28.5 ± 22.2 | 31.1 ± 21.9 | 0.757 |
| Bilirubin, g/dl | 0.8 ± 0.4 | 0.8 ± 0.5 | 0.9 ± 0.4 | 0.627 |
| Blood urea nitrogen, mg/dL | 14.8 ± 3.8 | 14.9 ± 3.5 | 14.5 ± 4.3 | 0.542 |
| Creatinine, mg/dL | 0.82 ± 0.15 | 0.77 ± 0.13 | 0.90 ± 0.16 | 0.007 |
| hs-CRP, mg/dL | 0.12 (0.03–0.26) | 0.04 (0.01–0.13) | 0.16 (0.07–0.27) | 0.008 |
Values are given as mean ± standard deviation or number (percentage), except for high-sensitivity C-reactive protein given as median (interquartile range).
ASCVD, Atherosclerotic Cardiovascular Disease; ACEI, angiotensin-converting enzyme inhibitors; ARB, angiotensin receptor blockers; LDL-C, low-density lipoprotein cholesterol; HLD-C, high-density lipoprotein cholesterol; hs-CRP, high-sensitivity C-reactive protein.
Table 2. Clinical and laboratory features before and after pitavastatin treatment.
| Baseline | 3 months | p value | |
|---|---|---|---|
| Clinical data | |||
| Systolic blood pressure, mmHg | 123 ± 11 | 125 ± 12 | 0.549 |
| Diastolic blood pressure, mmHg | 74 ± 10 | 75 ± 10 | 0.281 |
| Heart rate, bpm | 70 ± 11 | 68 ± 8 | 0.477 |
| Laboratory data | |||
| Total cholesterol, mg/dL | 246.5 ± 19.6 | 165.0 ± 27.3 | < 0.001 |
| LDL-C, mg/dL | 168.8 ± 19.7 | 89.9 ± 25.5 | < 0.001 |
| HDL-C, mg/dL | 56.0 ± 10.6 | 55.7 ± 11.8 | 0.834 |
| Triglyceride, mg/dL | 144.1 ± 67.3 | 137.3 ± 54.0 | 0.619 |
| hs-CRP, mg/dL | 0.12 (0.03–0.26) | 0.14 (0.04–0.26) | 0.256 |
Values are given as mean ± standard deviation or number (percentage), except for high-sensitivity C-reactive protein given as median (interquartile range).
LDL-C, low-density lipoprotein cholesterol; HLD-C, high-density lipoprotein cholesterol; hs-CRP, high-sensitivity C-reactive protein.
Effect of Pitavastatin on Carotid Ultrasound Parameters
Effects of high-dose pitavastatin on the carotid ultrasound variables are shown in Table 3. After 3 months of pitavastatin treatment, speckle-tracking-derived circumferential CAS significantly increased (2.98% ± 1.18% to 3.40% ± 1.43%, increase of 0.43% ± 1.13%, p = 0.008) and β2 significantly decreased compared with baseline (0.19 ± 0.07 to 0.17 ± 0.08, decrease of 0.02 ± 0.08, p = 0.047). There was no significant improvement in strain measured by B-mode ultrasound (10.13% ± 5.78% to 10.40% ± 4.68%, increase of 0.28% ± 6.11%, p = 0.644). No other conventional carotid elasticity metrics improved significantly after pitavastatin therapy. No significant changes were observed in mean carotid IMT and maximal plaque thickness from baseline. Fig. 3 shows the individual changes in carotid ultrasound measurements by 3 months of pitavastatin therapy.
Table 3. Carotid ultrasound parameters before and after pitavastatin treatment.
| Baseline | 3 months | p value | |
|---|---|---|---|
| Elasticity parameters by speckle-tracking method | |||
| Circumferential CAS, % | 2.98 ± 1.18 | 3.40 ± 1.43 | 0.008 |
| Stiffness index (β2) | 0.19 ± 0.07 | 0.17 ± 0.08 | 0.047 |
| Elasticity parameters by B-mode ultrasound | |||
| Strain (fractional diameter change), % | 10.1 ± 5.8 | 10.4 ± 4.7 | 0.644 |
| Stiffness index (β1) | 0.06 ± 0.03 | 0.06 ± 0.03 | 0.545 |
| Distensibility | 28.0 ± 14.3 | 31.6 ± 19.5 | 0.389 |
| Intima-media thickness, mm | 0.74 ± 0.16 | 0.73 ± 0.15 | 0.231 |
| Maximal carotid plaque thickness, mm | 1.99 ± 0.75 | 1.98 ± 0.58 | 0.786 |
Values are given as mean ± standard deviation.
CAS, carotid artery strain.
Fig. 3.
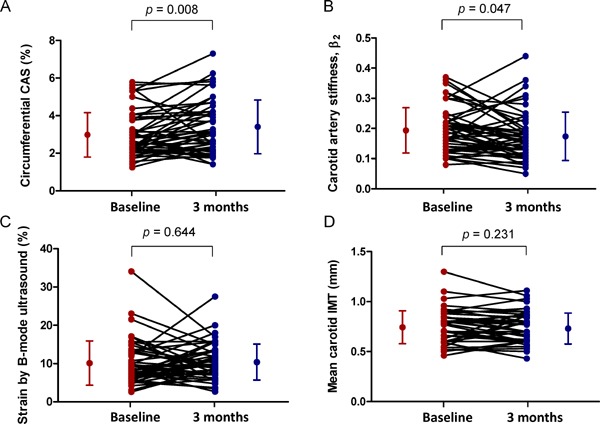
Plot of individual changes in carotid ultrasound variables by statin
The individual changes in circumferential CAS (A), stiffness index (β2) (B), strain by B-mode ultrasound (C), and mean carotid IMT (D) after high-dose pitavastatin therapy. The dots and bars on the sides indicate means and standard deviations.
CAS, carotid artery strain; IMT, intima–media thickness.
Differences in Statin Effects According to ASCVD Risk
When we stratified 48 patients into two subgroups according to the estimated ASCVD risk, LDL-C levels were significantly reduced with pitavastatin therapy in both patients with ASCVD risk ≥ 7.5% (decrease of −76.5 ± 25.2 mg/dL, p < 0.001) and those with ASCVD risk < 7.5% (decrease of −80.3 ± 18.9 mg/dL, p < 0.001). However, significant improvements in speckle-tracking-derived circumferential CAS (increase of 0.64% ± 1.17%, p = 0.045) and β2 from baseline (decrease of 0.04 ± 0.08, p = 0.028) were observed in patients with ASCVD risk ≥ 7.5%, but not in those with ASCVD risk < 7.5% (increase in circumferential CAS of 0.30% ± 1.11%, p = 0.076 and decrease in β2 of 0.01 ± 0.08, p = 0.417) (Fig. 4). There were no significant changes in conventional carotid elasticity metrics, mean IMT, and maximal carotid plaque thickness after pitavastatin therapy in both subgroups.
Fig. 4.
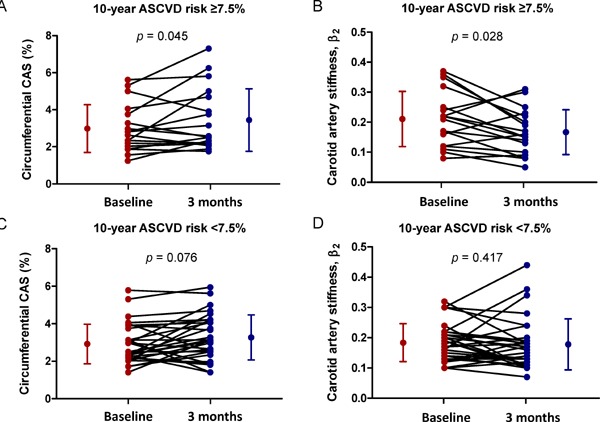
Plot of individual changes in speckle-tracking-derived carotid artery elasticity parameters according to 10 year ASCVD risk
Note significant improvements in circumferential CAS and β2 by speckle-tracking imaging in patients with ASCVD risk ≥ 7.5% (A and B), which are not observed in patients with ASCVD risk <7.5% (C and D). The dots and bars on the sides indicate means and standard deviations.
CAS, carotid artery strain; ASCVD, atherosclerotic cardiovascular disease.
Differences in Statin Effects According to Baseline LDL-C Levels
When dividing the patients into two groups according to the baseline LDL-C level (≥ 190 mg/dL versus < 190 mg/dL), CAS was significantly improved in both 6 patients (12.5%) with baseline LDL-C level ≥ 190 mg/dL and 42 patients (87.5%) with LDL-C < 190 mg/dL. The magnitude and significance of such improvement in CAS was greater in patients with LDL-C ≥ 190 mg/dL (+1.06 ± 1.00, p = 0.028) than those with LDL-C < 190 mg/dL (+0.34 ± 1.13, p = 0.046). With regard to β2, it was significantly improved in patients with LDL-C ≥ 190 mg/dL (−0.06 ± 0.06, p = 0.046), but not in those with LDL-C < 190 mg/dL (−0.01 ± 0.08, p = 0.163) (Supplemental Fig. 1).
Supplemental Fig. 1.
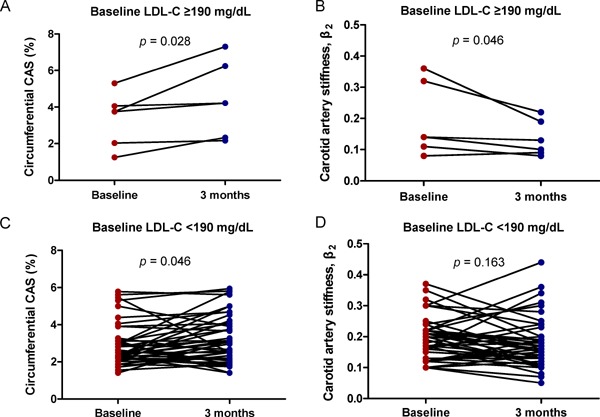
Plot of individual changes in speckle-tracking-derived carotid artery elasticity parameters according to baseline LDL-C levels
Note the effect of statin treatment on improvements in circumferential CAS and β2 was pronounced in patients with baseline LDL-C levels ≥ 190 mg/dL.
CAS, carotid artery strain; LCL-C, low-density lipoprotein cholesterol.
Measurement Reproducibility
Intraobserver and interobserver variabilities for circumferential CAS using speckle-tracking method were determined in all patients. Intraclass correlation coefficients (95% confidence interval) were 0.98 (0.96–0.99) for intraobserver variability and 0.97 (0.93–0.98) for interobserver variability, respectively. Bland–Altman plots also demonstrated low interobserver and intraobserver variabilities with no evidence of fixed or proportional bias (Fig. 5).
Fig. 5.
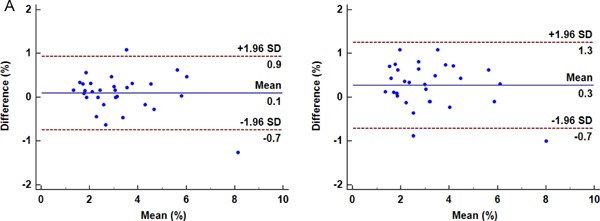
Bland–Altman analysis for circumferential CAS measurement
Bland–Altman plots of intraobserver (A) and interobserver variabilities (B) for measurements of circumferential CAS by speckle-tracking method. The solid line is the mean difference between the two scans, whereas the limits of agreement are drawn as dashed lines (mean difference ± 2 SD).
CAS, carotid artery strain; SD, standard deviation.
Discussion
The major findings of the current study are as follows: (1) short-term treatment with high-dose pitavastatin significantly improved carotid artery elasticity measured by speckle-tracking ultrasound, (2) conventional carotid elasticity parameters and mean IMT did not change after pitavastatin treatment, and (3) the measurement of circumferential CAS by speckle-tracking imaging was feasible and reproducible. These findings suggest that high-dose statin treatment exerts beneficial effects on vascular function in patients with hypercholesterolemia. Our study also implies that speckle-tracking imaging-based measurements may allow the early assessment of the potential vascular protective effects of statins.
Our results are in line with previous observations and strengthen the idea that statin therapy may have beneficial effects on arterial stiffness5, 6). Earlier basic research suggested biologically plausible mechanisms for the benefits of statin use on vascular function, including anti-inflammatory properties20), modulation of nitric oxide bioavailability21), and inhibition of LDL-C oxidation22). Several clinical studies proved that statin therapy can significantly improve vascular elasticity. In particular, many studies have focused on carotid arteries, because these are the most commonly used vascular beds for assessing early atherosclerosis in clinical practice. Specifically, a pilot study in statinnaïve subjects indicated that high-dose atorvastatin (80 mg daily) reduced carotid stiffness and increased distensibility5). Another study also showed that lowdose pitavastatin (1 or 2 mg daily) significantly improved the carotid artery elasticity in patients with hypercholesterolemia6).
Notably, aforementioned studies used classic arterial stiffness parameters from B-mode ultrasound to assess the effect of statin therapy. However, these parameters cannot directly measure the expansion of the intima–media wall, but rather the extension of the arterial wall according to the systolic and diastolic lumen diameter. For such reasons, conventional parameters have been suggested to have limited validity and reproducibility7, 16). Our study also supports this suggestion by showing that conventional parameters of carotid artery elasticity by B-mode ultrasound did not change after 3 months of high-dose pitavastatin treatment. Importantly, circumferential CAS and stiffness index (β2) measured by speckle-tracking imaging was significantly improved after pitavastatin treatment. These findings are not surprising given previous studies demonstrating that speckle-tracking imaging has a strong advantage in sensitivity compared with traditional modalities. For example, several studies have reported that speckle-tracking echocardiography is useful for early detection of subtle myocardial dysfunction in various conditions, including cancer chemotherapy, silent cerebrovascular disease, and valvular heart disease9–11), in whom traditional echocardiographic techniques do not adequately measure those changes. Speckle-tracking echocardiography has also been shown to have a higher sensitivity than conventional measures for detecting improvement of cardiac function after treatments, such as transcatheter pulmonary valve implantation12). Furthermore, speckle-tracking imaging has been recently applied to measure mechanical properties of carotid arteries7, 8), as the demand to have better imaging modalities for assessing carotid artery elasticity has become greater. Subsequently, it has been found that the ability of speckle-tracking-based strain analysis to detect agedependent differences in carotid elastic properties was superior to that of conventional measures16). In this respect, it may be inferred that speckle-tracking imaging provides more accurate assessment of arterial elasticity than B-mode ultrasound7).
It is also noteworthy that mean IMT and maximal plaque thickness, structural measures of atherosclerosis, did not significantly change after pitavastatin treatment. This finding is consistent with previous studies that have suggested no effect of statins on IMT or plaque regression5, 6, 23). Considering that a longer observation period is generally needed to observe the pharmacological effects on structural changes in atherosclerosis24), 3 month follow-up might be too short to assess the impact of statin on carotid IMT or atherosclerotic plaque burden. Hence, the assessment of carotid artery elasticity with speckle-tracking imaging can be useful in evaluating early effects of medical intervention.
Intriguingly, our study showed that significant improvements in circumferential CAS and β2 measured by speckle-tracking imaging were observed in patients with the estimated 10 year ASCVD risk ≥ 7.5%, but not in those with ASCVD risk < 7.5%. These findings are relevant given that the net benefit of statin therapy in reducing cardiovascular events is particularly well established in individuals with elevated ASCVD risk14). Moreover, the effect of statin treatment on improvements in circumferential CAS and β2 was pronounced in patients with baseline LDL-C levels ≥ 190 mg/dL (Supplemental Fig. 1). In this regard, our findings may provide further support for current guidelines recommending use of moderate-to-intensive statin therapy in patients with high ASCVD risk or high LDL-C levels14, 25). However, because there is lack of validated ASCVD risk estimation system specific to the Korean population, future investigations incorporating race-specific risk prediction model are required to confirm our findings.
Among various types of statins, pitavastatin was used in the present study, which has a comparable lipid-lowering efficacy to atorvastatin and rosuvastatin26, 27). Intriguingly, compared with other statins, pitavastatin has been reported to produce better metabolic profiles such as improved glucose metabolism28) and triglyceride control29). These observations suggest that the beneficial effects of pitavastatin on arterial elasticity may be maintained over a longer period of time, compared with other statins with adverse metabolic profiles30), although further studies are needed to validate this speculation31, 32).
Study Limitations
The present study has several limitations. First, the sample size was small and the study population was exclusively middle-aged Korean. Thus, the extrapolation of our data to other ethnic populations should be undertaken with caution. Second, there was no control group in this study, limiting our ability to adequately assess the effect of pitavastatin on study outcomes in comparison with other statins or placebo. Third, the elasticity parameters were calculated at a single site without plaque in the left common carotid artery, which could increase the variation in measurements of these parameters. However, intraobserver and interobserver variabilities were minimal in this study. Furthermore, great care was taken to select the same level of the common carotid artery at baseline and at follow-up. Fourth, considering that several clinical studies have reported anti-atherosclerotic effects of ACE inhibitors, ARB, or CCB33, 34), these medications should be considered as an adjusting covariate. Although chronic use of ACE inhibitors, ARB, or CCB without changes in the doses in our study could imply that their effects on carotid artery elasticity parameters might be less pronounced than new use or dosage change of these medications just before or during the study, their influence on carotid artery elasticity cannot be completely ruled out. This limitation warrants future studies assessing the effect of statins in conjunction with or in comparison with ACE inhibitors, ARB or CCB on carotid artery elasticity. Finally, this was a short-term observational study, which may create an evaluation bias. Therefore, further studies are required to confirm our findings as well as to determine their clinical significance.
Conclusions
The results of our study suggest that serial measurements of circumferential CAS and stiffness index (β2) using speckle-tracking imaging enable the evaluation of short-term effect of high-dose pitavastatin on carotid artery elasticity, whereas those of conventional parameters by B-mode ultrasound do not. These data lend preliminary support to the potential use of speckle-tracking imaging as a noninvasive tool for early assessment of treatment effect on vascular function in patients taking statin therapy.
Acknowledgements
The authors wish to thank Han Na Choi, RN, for taking care of the database for the present study and Su-Yeon Park and Oh Min Kwon for performing echocardiography and carotid ultrasound.
Funding
This study was supported by grants from Il-dong Pharmaceutical. Co., Ltd. (Republic of Korea).
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1). Zieman SJ, Melenovsky V, Kass DA: Mechanisms, pathophysiology, and therapy of arterial stiffness. Arterioscler Thromb Vasc Biol, 2005; 25: 932-943 [DOI] [PubMed] [Google Scholar]
- 2). Cheng KS, Baker CR, Hamilton G, Hoeks AP, Seifalian AM: Arterial elastic properties and cardiovascular risk/event. Eur J Vasc Endovasc Surg, 2002; 24: 383-397 [DOI] [PubMed] [Google Scholar]
- 3). Mercuro G, Zoncu S, Saiu F, Sarais C, Rosano GM: Effect of atorvastatin on endothelium-dependent vasodilation in postmenopausal women with average serum cholesterol levels. Am J Cardiol, 2002; 90: 747-750 [DOI] [PubMed] [Google Scholar]
- 4). John S, Schneider MP, Delles C, Jacobi J, Schmieder RE: Lipid-independent effects of statins on endothelial function and bioavailability of nitric oxide in hypercholesterolemic patients. Am Heart J, 2005; 149: 473. [DOI] [PubMed] [Google Scholar]
- 5). Ratchford EV, Gutierrez J, Lorenzo D, McClendon MS, Della-Morte D, DeRosa JT, Elkind MS, Sacco RL, Rundek T: Short-term effect of atorvastatin on carotid artery elasticity: a pilot study. Stroke, 2011; 42: 3460-3464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6). Mizuguchi Y, Oishi Y, Miyoshi H, Iuchi A, Nagase N, Oki T: Impact of statin therapy on left ventricular function and carotid arterial stiffness in patients with hypercholesterolemia. Circ J, 2008; 72: 538-544 [DOI] [PubMed] [Google Scholar]
- 7). Kim SA, Park SM, Kim MN, Kim YH, Cho DH, Ahn CM, Hong SJ, Lim DS, Shim WJ: The relationship between mechanical properties of carotid artery and coronary artery disease. Eur Heart J Cardiovasc Imaging, 2012; 13: 568-573 [DOI] [PubMed] [Google Scholar]
- 8). Teixeira R, Vieira MJ, Goncalves A, Cardim N, Goncalves L: Ultrasonographic vascular mechanics to assess arterial stiffness: a review. Eur Heart J Cardiovasc Imaging, 2016; 17: 233-246 [DOI] [PubMed] [Google Scholar]
- 9). Thavendiranathan P, Poulin F, Lim KD, Plana JC, Woo A, Marwick TH: Use of myocardial strain imaging by echocardiography for the early detection of cardiotoxicity in patients during and after cancer chemotherapy: a systematic review. J Am Coll Cardiol, 2014; 63: 2751-2768 [DOI] [PubMed] [Google Scholar]
- 10). Russo C, Jin Z, Homma S, Elkind MS, Rundek T, Yoshita M, DeCarli C, Wright CB, Sacco RL, Di Tullio MR: Subclinical left ventricular dysfunction and silent cerebrovascular disease: the Cardiovascular Abnormalities and Brain Lesions (CABL) study. Circulation, 2013; 128: 1105-1111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11). Gorcsan J, 3rd, Tanaka H: Echocardiographic assessment of myocardial strain. J Am Coll Cardiol, 2011; 58: 1401-1413 [DOI] [PubMed] [Google Scholar]
- 12). Chowdhury SM, Hijazi ZM, Rhodes JF, Kar S, Makkar R, Mullen M, Cao QL, Mandinov L, Buckley J, Pietris NP, Shirali GS: Changes in speckle tracking echocardiography measures of ventricular function after percutaneous implantation of the Edwards SAPIEN transcatheter heart valve in the pulmonary position. Echocardiography, 2015; 32: 461-469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13). James IC: Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel Ⅲ). JAMA, 2001; 285: 2486-2497 [DOI] [PubMed] [Google Scholar]
- 14). Stone NJ, Robinson JG, Lichtenstein AH, Goff DC, Jr., Lloyd-Jones DM, Smith SC, Jr., Blum C, Schwartz JS: Treatment of blood cholesterol to reduce atherosclerotic cardiovascular disease risk in adults: synopsis of the 2013 American College of Cardiology/American Heart Association cholesterol guideline. Ann Intern Med, 2014; 160: 339-343 [DOI] [PubMed] [Google Scholar]
- 15). Godia EC, Madhok R, Pittman J, Trocio S, Ramas R, Cabral D, Sacco RL, Rundek T: Carotid artery distensibility: a reliability study. J Ultrasound Med, 2007; 26: 1157-1165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16). Bjallmark A, Lind B, Peolsson M, Shahgaldi K, Brodin LA, Nowak J: Ultrasonographic strain imaging is superior to conventional non-invasive measures of vascular stiffness in the detection of age-dependent differences in the mechanical properties of the common carotid artery. Eur J Echocardiogr, 2010; 11: 630-636 [DOI] [PubMed] [Google Scholar]
- 17). Saito M, Okayama H, Inoue K, Yoshii T, Hiasa G, Sumimoto T, Nishimura K, Ogimoto A, Higaki J: Carotid arterial circumferential strain by two-dimensional speckle tracking: a novel parameter of arterial elasticity. Hypertens Res, 2012; 35: 897-902 [DOI] [PubMed] [Google Scholar]
- 18). Yang EY, Dokainish H, Virani SS, Misra A, Pritchett AM, Lakkis N, Brunner G, Bobek J, McCulloch ML, Hartley CJ, Ballantyne CM, Nagueh SF, Nambi V: Segmental analysis of carotid arterial strain using speckle-tracking. J Am Soc Echocardiogr, 2011; 24: 1276-1284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19). Oishi Y, Miyoshi H, Iuchi A, Nagase N, Ara N, Oki T: Vascular aging of common carotid artery and abdominal aorta in clinically normal individuals and preclinical patients with cardiovascular risk factors: diagnostic value of two-dimensional speckle-tracking echocardiography. Heart Vessels, 2013; 28: 222-228 [DOI] [PubMed] [Google Scholar]
- 20). Vlachopoulos C, Aznaouridis K, Dagre A, Vasiliadou C, Masoura C, Stefanadi E, Skoumas J, Pitsavos C, Stefanadis C: Protective effect of atorvastatin on acute systemic inflammation-induced endothelial dysfunction in hypercholesterolaemic subjects. Eur Heart J, 2007; 28: 2102-2109 [DOI] [PubMed] [Google Scholar]
- 21). Feron O, Dessy C, Desager JP, Balligand JL: Hydroxymethylglutaryl-coenzyme A reductase inhibition promotes endothelial nitric oxide synthase activation through a decrease in caveolin abundance. Circulation, 2001; 103: 113-118 [DOI] [PubMed] [Google Scholar]
- 22). Kleinveld HA, Demacker PN, De Haan AF, Stalenhoef AF: Decreased in vitro oxidizability of low-density lipoprotein in hypercholesterolaemic patients treated with 3-hydroxy-3-methylglutaryl-CoA reductase inhibitors. Eur J Clin Invest, 1993; 23: 289-295 [DOI] [PubMed] [Google Scholar]
- 23). David DM, Issam M, Mitchell SE, Sacco Ralph L, Tatjana R: The short-term effect of atorvastatin on carotid plaque morphology assessed by computerassisted grayscale densitometry: a pilot study. Neurol Res, 2011; 33: 991-994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24). Nozue T, Fukui K, Yamamoto S, Kunishima T, Umezawa S, Onishi Y, Tohyama S, Hibi K, Sozu T, Terashima M: Time course of statin-induced changes in coronary atherosclerosis using intravascular ultrasound with virtual histology. Coron Artery Dis, 2013; 24: 481-486 [DOI] [PubMed] [Google Scholar]
- 25). Catapano AL, Graham I, De Backer G, Wiklund O, Chapman MJ, Drexel H, Hoes AW, Jennings CS, Landmesser U, Pedersen TR, Reiner Z, Riccardi G, Taskinen MR, Tokgozohlu L, Verschuren WM, Vlachopoulos C, Wood DA, Zamorano JL: 2016 ESC/EAS Guidelines for the Management of Dyslipidaemias. Eur Heart J, 2016; 37: 2999-3058 [DOI] [PubMed] [Google Scholar]
- 26). Saku K, Zhang B, Noda K: Randomized head-to-head comparison of pitavastatin, atorvastatin, and rosuvastatin for safety and efficacy (quantity and quality of LDL)-The PATROL trial. Circ J, 2011; 75: 1493-1505 [DOI] [PubMed] [Google Scholar]
- 27). Hiro T, Kimura T, Morimoto T, Miyauchi K, Nakagawa Y, Yamagishi M, Ozaki Y, Kimura K, Saito S, Yamaguchi T: Effect of intensive statin therapy on regression of coronary atherosclerosis in patients with acute coronary syndrome: a multicenter randomized trial evaluated by volumetric intravascular ultrasound using pitavastatin versus atorvastatin (JAPAN-ACS [Japan assessment of pitavastatin and atorvastatin in acute coronary syndrome] study). J Am Coll Cardiol, 2009; 54: 293-302 [DOI] [PubMed] [Google Scholar]
- 28). Vallejo-Vaz AJ, Seshasai SRK, Kurogi K, Michishita I, Nozue T, Sugiyama S, Tsimikas S, Yoshida H, Ray KK: Effect of pitavastatin on glucose, HbA1c and incident diabetes: a meta-analysis of randomized controlled clinical trials in individuals without diabetes. Atherosclerosis, 2015; 241: 409-418 [DOI] [PubMed] [Google Scholar]
- 29). Teramoto T, Shimano H, Yokote K, Urashima M: Effects of pitavastatin (LIVALO tablet) on high density lipoprotein cholesterol (HDL-C) in hypercholesterolemia subanalysis of LIVALO effectiveness and safety (LIVES) study. J Atheroscler Thromb, 2009; 16: 654-661 [DOI] [PubMed] [Google Scholar]
- 30). Sattar N, Preiss D, Murray HM, Welsh P, Buckley BM, de Craen AJ, Seshasai SRK, McMurray JJ, Freeman DJ, Jukema JW: Statins and risk of incident diabetes: a collaborative meta-analysis of randomised statin trials. Lancet, 2010; 375: 735-742 [DOI] [PubMed] [Google Scholar]
- 31). Dei Cas A, Khan SS, Butler J, Mentz RJ, Bonow RO, Avogaro A, Tschoepe D, Doehner W, Greene SJ, Senni M: Impact of diabetes on epidemiology, treatment, and outcomes of patients with heart failure. JACC Heart Fail, 2015; 3: 136-145 [DOI] [PubMed] [Google Scholar]
- 32). Nakagomi A, Shibui T, Kohashi K, Kosugi M, Kusama Y, Atarashi H, Shimizu W: Differential effects of atorvastatin and pitavastatin on inflammation, insulin resistance, and the carotid intima-media thickness in patients with dyslipidemia. J Atheroscler Thromb, 2015; 22: 1158-1171 [DOI] [PubMed] [Google Scholar]
- 33). Hosomi N, Mizushige K, Ohyama H, Takahashi T, Kitadai M, Hatanaka Y, Matsuo H, Kohno M, Koziol JA: ACE inhibition with enalapril slows progressive intimamedia thickening of common carotid artery in non-insulin-dependent diabetes mellitus. Stroke, 2001; 32: 1539-1545 [DOI] [PubMed] [Google Scholar]
- 34). Nezu T, Hosomi N, Aoki S, Suzuki N, Teshima T, Sugii H, Nagahama S, Kurose Y, Maruyama H, Matsumoto M: Effects of cilnidipine, an L/N-type calcium channel blocker, on carotid atherosclerosis in Japanese post-stroke hypertensive patients: Results from the CA-ATTEND study. J Atheroscler Thromb, 2018; 25: 490-504 [DOI] [PMC free article] [PubMed] [Google Scholar]


