Abstract
Introduction
Tubular dysfunction is characteristic of Dent’s disease; however, focal segmental glomerulosclerosis (FSGS) can also be present. Glomerulosclerosis could be secondary to tubular injury, but it remains uncertain whether the CLCN5 gene, which encodes an endosomal chloride and/or hydrogen exchanger, plays a role in podocyte biology. Here, we implicate a role for CLCN5 in podocyte function and pathophysiology.
Methods
Whole exome capture and sequencing of the proband and 5 maternally-related family members was conducted to identify X-linked mutations associated with biopsy-proven FSGS. Human podocyte cultures were used to characterize the mutant phenotype on podocyte function.
Results
We identified a novel mutation (L521F) in CLCN5 in 2 members of a Hispanic family who presented with a histologic diagnosis of FSGS and low-molecular-weight proteinuria without hypercalciuria. Presence of CLCN5 was confirmed in cultured human podocytes. Podocytes transfected with the wild-type or the mutant (L521F) CLCN5 constructs showed differential localization. CLCN5 knockdown in podocytes resulted in defective transferrin endocytosis and was associated with decreased cell proliferation and increased cell migration, which are hallmarks of podocyte injury.
Conclusions
The CLCN5 mutation, which causes Dent’s disease, may be associated with FSGS without hyercalcuria and nepthrolithiasis. The present findings supported the hypothesis that CLCN5 participates in protein trafficking in podocytes and plays a critical role in organizing the components of the podocyte slit diaphragm to help maintain normal cell physiology and a functional filtration barrier. In addition to tubular dysfunction, mutations in CLCN5 may also lead to podocyte dysfunction, which results in a histologic picture of FSGS that may be a primary event and not a consequence of tubular damage.
Keywords: CLCN5, Dent's disease, FSGS, podocytes, renal biopsy
Dent’s disease, an X-linked inherited disease, is characterized by proximal tubule dysfunction that leads to end-stage renal disease (ESRD) in more than two-thirds of affected males. Mutations in the CLCN5 gene are responsible for 50% to 60% of cases.1 Close to 150 different CLCN5 mutations have been reported in patients with Dent’s disease.2, 3, 4 The CLCN5 gene encodes a chloride and/or proton exchanger that plays an important role in endosomal acidification and receptor-mediated endocytosis. The protein has 18 α-helices (A−R). More than 40% of mutations seen in Dent’s disease have been found in O and P helices.5
The clinical presentation of Dent’s disease may be deceptive, with a substantial number of patients expressing a partial or atypical phenotype,6 which causes difficulty in its diagnosis.2 Many patients may not have classical features (e.g., rickets, nephrocalcinosis, or nephrolithiasis) but may only have severe proteinuria, which consists of mostly low-molecular-weight proteins without high-grade albuminuria. On initial presentation, this high-grade proteinuria may be confused for nephrotic range proteinuria in patients with primary FSGS, when in fact, the underlying etiology is Dent’s disease; therefore, careful clinical evaluation is essential. Although Dent’s disease is largely considered a tubular disease,7 FSGS, or more commonly, focal global glomerulosclerosis (FGGS), may be seen as a dominant feature in some patients with Dent’s disease.7, 8
In the kidney, CLCN5 is expressed in proximal tubules, thick ascending limbs, and α-intercalated cells of the collecting duct.9 The protein functions as a 2 Cl−/H+ exchanger and is involved in the acidification of endosomes, processing, and degradation of absorbed proteins, and megalin-dependent absorption of proteins. The expression of CLCN5 in glomerular cells has not been well-documented. Therefore, it is intriguing that the glomerular pathology is caused by a variant of a tubular protein.
A key aspect of primary FSGS pathogenesis is podocyte damage and loss.10, 11 Mutations in genes that encode glomerular proteins, particularly in visceral epithelial cells (podocytes), lead to the development of FSGS.12 Previous reports have suggested that primary tubular injury may lead to glomerular sclerosis by mechanisms that are not yet understood.13, 14 In this study, we show that a variant of CLCN5 is present in a family with FSGS, and that CLCN5 is expressed in human podocytes and may play a role in glomerular physiology and pathology. Based on our results, we hypothesize that FSGS lesions, which are observed in patients with Dent’s disease, result from altered localization and/or function of CLCN5 in the podocytes, and are not purely a secondary consequence of tubular injury. This novel mutation has provided a unique opportunity to explore the mechanism by which the 2 Cl−/H+ exchanger functions in podocytes.
Materials and Methods
The study was approved by the Medical University of South Carolina (MUSC) Institutional Review Board, and signed informed consent was obtained from all study participants. Urine calcium was measured using Abbott Architect analyzer (Abbott Park, IL) at the MUSC central laboratory, and urine β2-microglobulin at the ARUP Laboratory (Salt Lake City, Utah) using a quantitative chemiluminescent immunoassay. Whole blood was collected from affected and unaffected family members in purple top ethylenediamine tetraacetic acid tubes.
Whole Exome Capture and High-Throughput Sequencing
DNA was extracted from the blood of the individuals using standard protocols. The DNA was exome-enriched, followed by high-throughput sequencing. Enriched libraries were prepared using Agilent’s (Santa Clara, CA) Sure Select XT Human All Exon V5+UTRs library kit for the Illumina platform (Illumina, San Diego, CA). Adapters were ligated to sheared DNA followed by hybridization to baits for a 75-Mb exome capture. Sequencing was performed on the captured exomes following the manufacturer’s protocol using 125 bp paired-end sequencing on an Illumina HiSeq2500, using version 4 reagents and software. Data for each sample was obtained to ensure an overall average of 100× coverage. Fastq file output was used for downstream bioinformatics analysis.
Bioinformatics Analysis of Whole Exome Sequencing Data (Data Analysis and Statistical Justification)
Paired-end (2 × 125 bases) DNA sequence reads that passed the Illumina quality control step were included in downstream analysis. Alignment and variant calling was performed using MiSeq Reporter Software version 2.4 (MSR) (Illumina) with GRCh37 Genome Reference Consortium Human Build 37/HG19 as the reference genome. Variant analysis was performed with VariantStudio version 2.2.174 (Illumina). Filtering of variant call files (vcf) files was carried out as follows: variants that passed the filter that incorporated all variants types were examined. VariantStudio settings were as follows: quality was required to be >100, read depth was >10, and all population frequencies were <5%. X-linked was checked using the family-based filtering option. Only variants mapped to a single site in all affected family members were selected for further analysis. Initial analysis focused on variants that were mapped to the X chromosome because the pedigree suggested an X linkage. Sequence variants that were homozygous in the 2 affected family members and heterozygous or absent in the 3 unaffected family members were identified, as well as the allele frequency of each variant obtained from the dbSNP version 138 reference database. The dbSNP database was then used to assign the value of “probable pathogenic” when appropriate. Those variants present in genes previously implicated in FSGS were selected for further study. Although the cohort we examined was small, the apparent X-linkage improved the chance to identify a pathologic variant. All phenotypic information and data from fastq and vcf were submitted to the National Center for Biotechnology Information database of genotypes and phenotypes (accession number pending).
In silico Functional Prediction of Mutations
Functional prediction of the CLCN5 mutation was performed using the online in silico prediction software packages, PolyPhen-2 (http://genetics.bwh.harvard.edu/pph2), SIFT (http://sift.bii.a-star.edu.sg), and MutationTaster (http://www.mutationtaster.org).
Cell Culture and Histochemistry
The pCMV6-AC-GFP vector carrying the wild-type CLCN5 gene (WT_CLCN5) and L521F mutant pCMV6-AC-GFP vector (L521F_CLCN5) were purchased from Origene (Rockville, MD). Sequencing of a constructed vector containing the L521F mutation confirmed the veracity of the mutation. Both cDNAs were in the frame so that each protein would be fused to green fluorescent protein (GFP). HEK293 cells were purchased from ATCC. HK-2 and HEK293 cells were grown in DMEM/F12 199/EBSS medium (Gibco, Life Technologies), respectively, until cells reached 70% confluence. Cells were transiently transfected with vectors using Lipofectamine 2000 transfection agent following the manufacturer’s instructions. Twenty-four hours (HEK293 cells) or 48 hours (HK-2 cells) later, images were taken using confocal microscopy. Double staining for lysosome and CLCN5 proteins were done. In brief, cells were grown as described previously, and after transfection for 24 hours, they were incubated with Lysotracker deep red dye (Life Technologies, Cat. No. L12492) for 2 hours. Images of live cells were taken using immunofluorescence microscopy (Leica Microscope, DMI 4000B). The human podocyte cell line was cultured in Roswell Park Memorial Institute (RPMI) 1640-based medium supplemented with 10% fetal bovine serum (Corning), insulin-transferrin-selenium (ITS) supplement (Sigma-Aldrich), 200 U/ml penicillin, and streptomycin (Roche Applied Science), as described previously.15 Human kidney sections were obtained from the MUSC tissue bank. In brief, normal renal tissue was obtained from nephrectomy specimens and embedded in paraffin. Five-micrometer sections were cut and processed.
ShRNA-based Knockdown of CLCN5 Gene in Human Podocytes Cell Line
To target CLCN5, we screened Mission Lentiviral Transduction particle shRNAs in pLKO.1-puro (CLCN5 MISSION shRNA-particle, commercially purchased from Sigma, catalog number SHCLNG-NM_000084; TRC number: TRCN0000043903, TRCN0000043904, TRCN0000043905, TRCN0000043906, TRCN0000043907, TRCN000414058, and TCRN000427059; each shRNA are designated as 903, 904, 905, 906, 907, 058, and 059, respectively, in the present study). Transfection of the ShRNA plasmids into the human podocyte cell line was performed with Lipofectamine 2000 (Invitrogen, Carlsbad, CA) according to the manufacturer's protocol. Transfected podocytes cells were grown in 2.5 μg/ml puromycin containing medium for the selection of stable transfectants and the CLCN5 knocked down was confirmed by Western blot.
Immunoblot Analysis
Immunoblotting experiments were done as described previously.15 A human podocyte cell line with CLCN5 knockdown was plated and allowed to grow at 33°C. The cells were harvested 48 hours later and rinsed twice with phosphate-buffered saline. Cell lysate was made using Radio immunoprecipitation assay buffer (buffer/lysis buffer). The membrane was immunoblotted with antichloride channel CLC-5 antibody produced in rabbits (C1116, Sigma-Aldrich), 1:500 dilution in TBST with 3% bovine serum albumin (Sigma Aldrich, St Louis, MO). To assure equivalent protein loading, the membranes were simultaneously incubated with glyceraldehyde-3-phosphate dehydrogenase monoclonal antibody from Sigma (1:10,000) at 4°C overnight. Membranes were washed 3 times with TBST, incubated with horseradish peroxidase−conjugated secondary antibodies (Pierce) at 1:10,000 dilutions for 1 hour at room temperature, and washed extensively before detection. The membranes were subsequently developed using Super Signal West Femto Maximum Sensitivity Substrate (Pierce, Cat. No. 34095), and images were collected with a LICOR Image analyzer.
Endocytosis
Internalization assays using Transferrin from Human Serum, Alexa Fluorconjugated (Molecular Probes, Cat. No. T23365) were performed as described16, 17 with some modifications. Citrate buffer (pH: 2.5) was used to remove surface-bound transferrin. Images were collected at 10, 15, and 30 minutes postincubation using a Leica confocal microscope (TCS SP5). For the analysis of transferrin uptake in cells where CLCN5 was knocked down, images of at least 50 surface-bound transferrin cells were collected for 2 clones and a corresponding control. Cells that exhibited punctate transferrin labeling were counted as positive, and cells that did not contain distinct transferrin puncta were counted as negative.
Migration and/or Wound Assay
A migration assay was done as described previously with minor modification.18, 19 Control and CLCN5 knockdown podocytes were grown in 35-mm glass-bottom culture dishes (MatTek Corporation) until a confluent cell monolayer was achieved. The cells were serum-starved in RPMI 1640 medium for 8 to 12 hours. A scratch wound was created using a 1- to 10-μl pipette tip. Wounds were created with 2 strokes at a 90-degree angle and washed twice with phosphate-buffered saline to remove all the suspended cells in the medium. The cells were then cultured in RPMI 1640 medium supplemented with 10% fetal bovine serum, ITS supplement (Sigma-Aldrich), and 200 U/ml penicillin and streptomycin (Roche Applied Science) at 33°C for 12 hours. Images were taken at different time points (0, 6, and 10 hours). The experiment was performed 3 times, and the rate of migration was calculated using ImageJ software.
Cell Proliferation Assay
An equal number (50,000 cells) of control and CLCN5 knockdown podocytes were plated and allowed to grow for 24, 48, or 72 hours. To measure the difference in the rate of proliferation, the cells were trypsinized, and cells in suspension were counted using a hemocytometer. The number of cells at different time points was plotted, and differences between the control and CLCN5 knockdown clones were calculated.
Immunofluorescence Microscopy
Cultured podocytes were grown on coverslips as described previously.20 Each experiment was carried out in 3 independent sets, and the images were collected using a Leica immunofluorescence Microscope (Lieca DMI 400B).
Statistical Analysis
Statistical analysis was performed using GraphPad PRISM 7.01 software. Distribution of cell wound closure data normality was tested through the D'Agostino and Pearson normality test and passed this test, whereas the Shapiro-Wilk normality test showed all the data used for the cell proliferation analysis had a normal distribution. Statistical significance was determined by 2-way analysis of variance, and P values were adjusted using Tukey’s multiple comparison. P values <0.05 was considered statistically significant. Mean fluorescence intensity was measured by immunofluorescence images using ImageJ software (National Institutes of Health, Bethesda, MD), and >50 images were used from 3 different experiments. Mean pixel intensity differences between control and CLCN5 knockdown podocytes were analyzed through the nonparametric Mann-Whitney test, and P < 0.05 was considered statistically significant.
Results
Pedigree Analysis and Whole Exome Sequencing
The proband, a 37-year-old Hispanic man, was evaluated by a local nephrologist in Guadalajara, Mexico for proteinuria and an increase in serum creatinine. Based on the kidney biopsy (Figure 1a), he was diagnosed with FSGS in 2012. Two years later, in January 2014, he presented to our hospital for kidney transplantation. During a donor evaluation at that time, it was revealed that his 32-year-old brother had similar symptoms, and based on a kidney biopsy (Figure 1b) was also diagnosed with FSGS. Based on a survey of both affected brothers, we created a pedigree and identified 9 family members in total who had kidney disease. The pedigree presented in Figure 1c is suggestive of an X-linked mode of inheritance. We obtained whole blood and performed whole exome sequencing in 6 individuals: the proband, 1 affected brother, 1 nonaffected brother, an affected uncle, and the proband’s nonaffected mother and sister. Urine was obtained from the proband’s sister, mother, clinically nonaffected brother, and affected brother, who did not yet have ESRD. Total calcium and β2 microglobulin excretion values are presented in Table 1. The excretion of β2 microglobulin (corrected for urine creatinine) was >2000 times greater for the affected brother compared with the nonaffected brother and sister; whereas in the unaffected carrier mother, β2 microglobulin excretion (corrected for creatinine) was only approximately 64 times greater.
Figure 1.
Identification of patients with focal segmental glomerulosclerosis (FSGS) and pedigree analysis. We identified 2 brothers, the proband (a) and the affected brother (b) who presented to a local nephrologist, in Mexico, with proteinuria and altered renal function. The silver-stained biopsy image shows a segmentally sclerotic glomerulus. Other areas of the glomeruli appear relatively normal. Both were diagnosed with FSGS based on kidney biopsies presented here. Pedigree of the family with X-linked inheritance of FSGS (c). The red arrow indicates the proband. Blood was collected from the proband and 5 additional family members indicated by asterisks. The pedigree suggested an X-linked pattern of inheritance. The open boxes and circles represent the normal males and females, respectively. The closed boxes indicate the affected male, and the crossed-out boxes indicate the deceased family members.
Table 1.
Urine laboratory values in affected and unaffected family members
| Parameter | Nonaffected brother | Affected brother | Carrier sister | Carrier mother |
|---|---|---|---|---|
| Spot urine calcium (mg/dl) | 10.5 | 6.0 | 22.4 | 3.9 |
| Spot urine creatinine (mg/dl) | 160.7 | 46.2 | 97.5 | 72.4 |
| Calcium creatinine ratio (mg/mg) | 0.06 | 0.12 | 0.22 | 0.05 |
| Spot urine β2-MG (μg/l) | 71 | 42,653 | 22 | 1184 |
| Spot urine creatinine (mg/dl) | 152 | 46 | 79 | 77 |
| β2-MG creatinine ratio (μg/g) | 47 | 96,939 | 28 | 1538 |
Normal value for calcium creatinine ratio <025. Reference value for β2-microglobulin (β2-MG) creatinine ratio 0 to 300. Small differences in spot urine creatinine are because the β2-MG assays were performed at the ARUP Laboratory and the calcium assay at the MUSC Laboratory.
Whole exome sequencing identified 6 gene variants in 5 genes on the X chromosome in the proband: CLCN5 (NM_001127898.1:c.1771C>T), HDX (NM_144657.4:c.1190T>C), KIAA2022 (2 variants: NM_001008537.2:c.3001G>C, NM_001008537.2:c.2851G>A), SLC16A2 (NM_006517.4:c.538G>A), and SSX5 (NM_021015.3:c.337C>T). Of the 6 candidate gene variants, only 1 was present in all affected members, which demonstrated heterozygosity in the proband’s mother and sister (i.e., only 1 X chromosome affected), and was not present in the nonaffected brother. Based on this rationale, the most likely candidate responsible for the development of FSGS in this family was the mutation found in the CLCN5 gene. The mutation involves replacement of phenylalanine for leucine at position 521 (L/F521, CLCN5 variant 3 NM_000084.4) in the shorter variant or position 591 (L/F591, CLCN5 variant 1 NM_001127899.3) in the longer variant.
In silico Prediction of the Effect of Amino Acid Change on CLCN5
Polyphen-2, SIFT, and MutationTaster servers were used to predict the effect of mutation L521F on the CLCN5 gene. Based on the output obtained using server Polyphen-2, the mutation was predicted to be damaging, with a score of 0.884 (sensitivity: 0.82; specificity: 0.94). Interestingly, using the server SIFT, substitution at position 521 from L to F was predicted to be intolerant, and could have affected protein function, with a score of 0.04. The MutationTaster server also predicted that the mutation would affect protein physiology, leading to a disease phenotype.
Cellular Localization of Wild-type and Mutant CLCN5-L521F Proteins
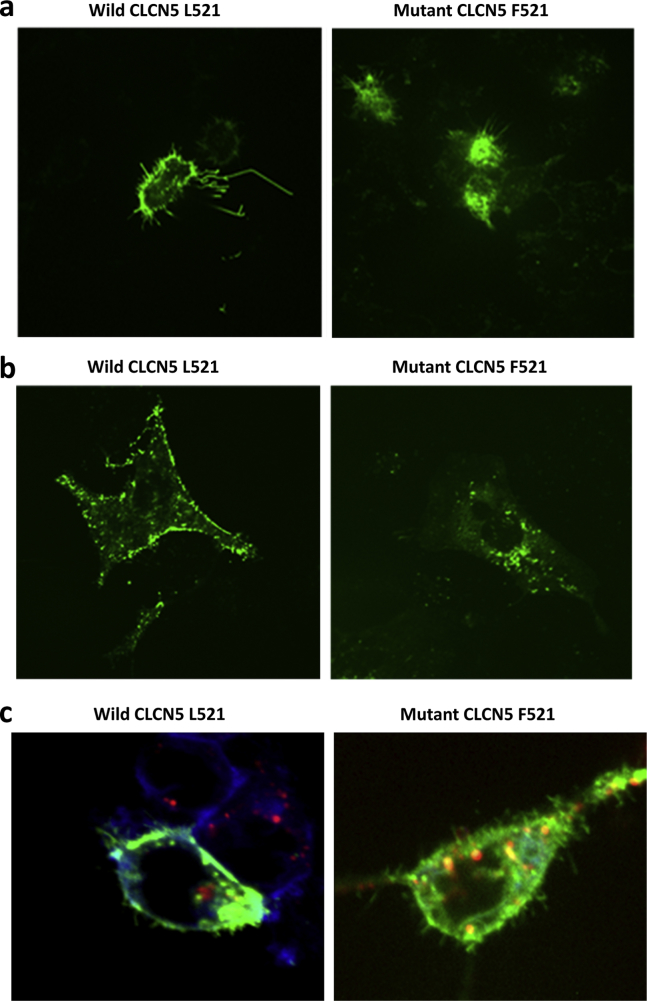
To further analyze if the L521F mutation resulted in defective cellular localization of CLCN5 protein, we overexpressed these genes in cultured HEK and HK-2 cells. Cells were transiently transfected with the pCMV6-AC-GFP vector carrying wild-type CLCN5 cDNA (WT_CLCN5) and the L521F mutant pCMV6-AC-GFP vector (L521F_CLCN5). As seen in Figure 2a (left panel), HEK cells transfected with WT_CLCN5 showed significant localization of CLCN5 protein at the cell membrane. In contrast, HEK cells transfected with L521F_CLCN5 showed expression of the mutant L521F CLCN5 protein primarily in the cytoplasm (Figure 2a, right panel). Similar findings were noted in the HK-2 transfected cells, as demonstrated in Figure 2b (left and right panels for the wild-type CLCN5 and L521F mutant, respectively).
Figure 2.
Transient expression of wild-type (WT) and L521F mutant CLCN5-GFR constructs in HEK cells and HK-2 cells. In HEK cells, WT CLCN5 protein is predominantly expressed on the cell membrane (left panel), whereas the L591F mutant displayed cytoplasmic localization (right panel) (a). In HK-2 cells, WT CLCN5 protein is predominantly expressed on the cell membrane (left panel), whereas the L591F mutant displays cytoplasmic localization (right panel) (b). Intracellular localization of WT and mutant CLCN5 in human podocytes (c). The right panel shows mutant CLCN5 protein that displays greater colocalization within lysosomes and disperses cytoplasmic distribution compared with a primarily membrane distribution in the WT CLCN5 podocytes (left panel).
To further investigate if L521F CLCN5 was entrapped in lysosomal structures, we used a lysosome-specific stain, Lysotracker deep red. Figure 2c shows colocalization of GFP-labeled CLCN5 protein (green) with the lysosomal-specific marker (red). As demonstrated in Figure 2c, compared with the wild-type CLCN5 protein (left panel), the mutant L521F CLCN5 protein showed increased co-localization with lysosomes (right panel). These findings were consistent with previous reports that showed that the CLCN5 (L521F) mutation resulted in the formation of a defective protein that was aberrantly processed and transferred to lysosomes for degradation.
Identification of CLCN5 Protein in Cultured Human Podocytes and Kidney Sections
A single laboratory group demonstrated that CLCN5 is expressed in glomerular podocytes, in which CLCN5 belongs to a complex of proteins involved in albumin reabsorption in the podocytes21, 22; however, these data have not been independently verified, and the effect of CLCN5 mutations in podocytes have not been studied. Figure 3a shows a Western blot of homogenized human cultured podocyte lysate probed with an anti-CLCN5 antibody. We identified a single band corresponding to the approximate molecular weight of 80 kDa, which most likely represented the shorter, 746 amino acid CLCN5 proteoform. Immunofluorescence studies presented in Figure 3b further supported the Western blot findings and demonstrated the expression of endogenous CLCN5 in human cultured podocytes. We further tested glomerular expression in sections of normal mouse and human kidney, and found significant expression of the CLCN5 protein in mice kidney glomeruli and parietal epithelial cells.
Figure 3.
Expression of CLCN5 constructs in human podocyte cell lines. Western blot (WB) showed a single band consistent with the short 83-kDa CLCN5 proteoform. Neph1 expression, which was used a positive control, was confirmed using an anti-Neph1 antibody (Ab; a). Endogenous expression of CLCN5 was also determined by immunofluorescence in cultured human podocytes (b). The upper panel shows human podocytes transfected with green fluorescent protein (GFP)–tagged wild-type (WT) CLCN5 protein demonstrated predominantly cell surface distribution and co-localization with cell surface marker ZO-1. The lower panel shows cultured human podocytes transfected with GFP-tagged L521F mutant CLCN5 protein, which demonstrated predominantly intracellular distribution (c). DAPI, 4′,6-diamidino-2-phenylindole.
Cellular Localization of Wild-type and L521F Mutant CLCN5 Protein in Cultured Human Podocytes
A cultured human podocyte cell line was transfected with wild-type CLCN5 (WT_CLCN5) and mutant CLCN5 (L521F_CLCN5) constructs. As demonstrated in Figure 3c, cells transfected with a GFP-tagged, wild-type construct demonstrated both cytoplasmic and cell surface distribution of the protein; in contrast, the cultured human podocytes transfected with GFP-tagged L521F CLCN5 demonstrated predominantly intracellular distribution. Importantly, the results obtained with human podocytes were similar to the observations noted in the transfected human renal proximal tubule cells.
Effect of Knockdown of CLCN5 Expression on Cell Proliferation−Cell Migration in Podocyte Cells
To further examine the involvement of CLCN5 in trafficking mechanisms in podocytes, especially in endocytosis, we generated a stable human podocyte cell line in which the CLCN5 gene was knocked down using the CLCN5 specific shRNA. The CLCN5 knockdown in podocyte cell lines was confirmed by immunoblotting (Figure 4a, upper panel). The cell proliferation assay (Figure 4a, lower panel) showed a reduced rate of proliferation in the CLCN5 knockdown human podocytes compared with the control podocytes. Interestingly, the rate of cell migration, as assessed by a scratch assay, in CLCN5 knockdown cells was increased compared with the control podocyte cells (Figure 4b). The increased rate of cell migration was reported as abnormal and was a sign of podocytes injury.
Figure 4.
shRNA-based knockdown of CLCN5 in human podocytes. Western blot showing CLCN5 knockdown in human podocytes using 7 different shRNA clones (a). Cell proliferation assay showed that CLCN5 knockdown podocytes have a reduced cell proliferation rate compared with control. The rate of clone 1 was depressed at 24 hours, and the rate of clone 2 was depressed at 72 hours (P = 0.01 and 0.004, respectively). All data are presented as mean ± SD (n = 4) (b). Wound closure assay showed that both clone 1 and clone 2 have a reduction in wound closure at 6 hours (P = 0 .002 and 0.05, respectively) and 10 hours (P < 0.001 and 0.02, respectively). All data are presented as mean ± SD (n = 8) (c). Endocytosis: internalization assay using Alexa-568-transferrin were performed. The images collected after 10, 20, and 30 minutes are shown, which suggests defective endocytosis in the CLCN5 knockdown in the human podocytes cell line. CLCN5 knockdown podocytes displayed a significant reduction in transferrin endocytosis at all time points (P < 0.001, respective control vs. respective knockdown, Mann-Whitney U test). All data are presented as mean ± SD (n = 50, images per group) (d). AU, arbitrary units; DAPI, 4′,6-diamidino-2-phenylindole; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; KD, knocked down.
Endocytosis
CLCN5 was shown to play a role in the uptake of low-molecular-mass proteins through receptor-mediated endocytosis in proximal tubules.23 It was also reported that CLCN5 channel disruption impaired endocytosis in a mouse model for Dent’s disease.24 To examine whether CLCN5 was involved in endocytosis in human podocytes, we monitored uptake of transferrin (Alexa Fluor conjugated) in CLCN5 knockdown human podocyte cells. As shown in Figure 4c (left and right panels) and Figure 4d, the fluorescence intensity of endocytosed transferrin was much higher in wild-type human podocytes compared with the CLCN5 knockdown cells after 10, 20, and 30 minutes of incubation. These results suggested that CLCN5 played a key role in trafficking mechanisms in podocytes.
Discussion
In this study, we identified and characterized a novel mutation in the CLCN5 gene found in a Hispanic family whose 2 members presented with overt proteinuria and were diagnosed with FSGS. None of the family members were aware of or had classical features of Dent’s disease (e.g., nephrocalcinosis, nephrolithiasis, rickets, and hypercalciuria). Proteinuria on the initial evaluation was not characterized as consisting of only low-molecular-weight proteins. According to the literature,3, 7, 25 erroneous diagnosis and treatment of presumptive FSGS is not a rare event because a considerable number of patients with Dent’s disease, like those in our study, have an oligosymptomatic phenotype.
CLCN5 protein has 2 predominant splice proteoforms: a shorter form consisting of 746 amino acids (molecular weight 83 kDa; Uniprot accession number P51795-1), and a longer form that includes an additional 70 amino acids at the N-terminal end, thereby creating an 816 amino acid protein (molecular weight 90 kDa; Uniprot accession number P51795-2). The mutation described resulted in the replacement of leucine with phenylalanine at position 521 (short variant, Uniprot accession number P51795-1) or position 591 (long variant, Uniprot accession number P51795-2). Based on our findings, we postulated that this mutation had pathogenic significance. First, the mutation on this specific site, which involved replacement of leucine with arginine (L521R), was previously reported in a pediatric patient with Dent’s disease,26 but there were no studies with respect to the consequence of CLCN5 mutations on the function of human podocytes that might underlie the observed glomerular involvement. Second, the experimental replacement of leucine with arginine at position 521 resulted in defective trafficking to the surface of Xenopus oocytes compared with wild-type CLCN5, which suggested mutations at this site could impair localization to the plasma membrane.27 Third, the mutation was located in the P helix of the CLCN5 protein, a region that is highly conserved across the members of the CLCN family.5 The CLCN5 exchanger functions as a homodimer, and integrity of the P helix is essential for homodimer formation.28 Our results were consistent with the previous report, which found that mutations at the leucine 521 position resulted in improper trafficking of the mutant protein and might be directed to lysosomes for degradation as observed from the Lysotracker staining experiments in our study (Figure 2c).
The lesions of FSGS and FGGS have increasingly been reported in patients with Dent’s disease. In the most extensive study published so far, which involved 30 kidney biopsies, FGGS was found in 83.3% of cases, FSGS in 6.6%, and segmental capillary collapse in 6.6% of cases. Segmental foot process effacement was found in all cases, but was analyzed in less than one-half of biopsy samples.29 The appearance of glomerulosclerosis in a patient with Dent’s disease has generally been regarded as a consequence of tubular damage; however, no plausible mechanism has been proposed.2, 3, 26 Based on data presented within this study, we postulated that a mutation in CLCN5 could lead to glomerulosclerosis, in part, through primary podocyte injury.
To evaluate the role of CLCN5 in podocyte damage, we first demonstrated that CLCN5 was expressed in cultured human podocytes both by Western blotting and immunohistochemistry. The physiological role of CLCN5 in podocytes was not previously investigated, although its expression was shown in podocytes.22 Evidence suggested that similar to proximal tubular cells, endocytic mechanisms operate in podocytes and play an important role in the maintenance of the glomerular filtration barrier.30 Furthermore, it appeared that glomerular expression of CLCN5 was increased in some proteinuric patients.21
Our experiments demonstrated that the identified human mutation (L521F) led to altered subcellular localization of the CLCN5 protein in podocytes in the same manner described for cultured human proximal tubular cells.31 It was thus conceivable that CLCN5 might have a similar role in podocytes as in proximal tubular cells. Data from immortalized proximal tubule cell lines derived from patients with Dent’s disease indicated that similar abnormalities resulting from CLCN5 mutations might be derived through differing cellular phenotypes affecting either endosomal acidification and/or receptor-mediated endocytosis.31 We demonstrated impaired endocytosis in cultured podocytes with genetic knockdown of CLCN5 genes.
Previous studies demonstrated that in response to direct cell injury, cultured podocytes changed from limited motility to enhanced motility and loss of stress fibers.18 Upon insult, stationary podocytes upregulated cytosolic cathepsin L expression and activity, and developed motile podocyte foot processes. This migratory response led to foot processes effacement, slit diaphragm remodeling, and proteinuria.31, 32, 33 This observation suggested that stress-induced podocyte motility associated with actin dynamics resulted in dysfunction of slit molecules and the integrin network. We demonstrated that deletion of the CLCN5 gene in cultured podocytes resulted in increased cell migration, which is an established phenotype of injured podocytes. These data, together with our finding of impaired endocytosis, suggested that the presence of CLCN5 is necessary for proper podocyte function. Recognition of the crucial role of CLCN5 in podocyte protein trafficking holds promise for the identification of important molecular events that regulate podocyte injury.
Collectively, the results presented in this study suggested that CLCN5 is expressed in podocytes and plays a critical role in podocyte function by participating in protein endocytosis. We provided some evidence that FSGS or FGGS observed in Dent’s disease might be the result of primary podocyte injury independent of tubular injury. However, at this time, we cannot conclude that the mutation presented here has pathological consequences for podocytes. The concept that a condition, which has historically been considered primarily tubular in origin, can lead to FSGS does not appear to be limited to CLCN5 mutations. Recently, lesions of FSGS were seen in patients with a mutation in the TTC21B gene, which encodes a ciliary protein and causes nephronophthisis, a classical tubular disease. Similar to CLCN5, the expression of TTC21B was detected in podocytes.34, 35 The probability that Dent’s disease is also a podocyte disease raises intriguing questions regarding the use of angiotensin-converting enzyme inhibitors or angiotensin receptor blockers for individuals with this condition. The use of enalapril and candesartan were reported in isolated case reports of Dent’s disease,2, 36 but never tested systematically. Based on our observations, we believe placebo-controlled trials using angiotensin antagonists would be highly recommended to assess their impact on the progression of Dent’s disease. In addition, CLCN5 knockout mice that replicate Dent’s disease have been generated and may serve as a useful animal model to assess the effect of angiotensin blockade on disease progression.
For physicians treating patients with a pathological diagnosis of FSGS and proteinuria, it is of utmost importance to make sure that low-molecular-weight proteinuria and Dent’s disease are excluded before subjecting patients to toxic immunosuppressive treatment, because Dent’s disease with glomerular involvement is no longer considered a rare event.3, 7, 25
Disclosure
All the authors declared no competing interests.
Acknowledgments
The authors thank the American Society of Nephrology for the Ben J. Lipps Research Fellowship to AKS. This work was supported in whole or in part by an National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases Grant, 2R01DK087956-06A1 to DN and Dialysis Clinics Incorporated research funds to MB and MJ.
Contributor Information
Deepak Nihalani, Email: nihalani@musc.edu.
Milos N. Budisavljevic, Email: budisamn@musc.edu.
References
- 1.van Barkel Y., Ludwig M., van Wijk J.A.E. Proteinuria in Dent disease: a review of the literature. Pediatr Nephrol. 2017;32:1851–1859. doi: 10.1007/s00467-016-3499-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Copelovitch L., Nash M.A., Kaplan B.S. Hypothesis: Dent disease is an underrecognized cause of focal glomerulosclerosis. Clin J Am Soc Nephrol. 2007;2:914–918. doi: 10.2215/CJN.00900207. [DOI] [PubMed] [Google Scholar]
- 3.Frishberg Y., Dinour D., Belostotsky R. Dent's disease manifesting as focal glomerulosclerosis: is it the tip of the iceberg? Pediatr Nephrol. 2009;24:2369–2373. doi: 10.1007/s00467-009-1299-2. [DOI] [PubMed] [Google Scholar]
- 4.Kubo K., Aizawa T., Watanabe S. Does Dent disease remain an underrecognized cause for young boys with focal glomerulosclerosis? Pediatr Int. 2016;58:747–749. doi: 10.1111/ped.12944. [DOI] [PubMed] [Google Scholar]
- 5.Wu F., Roche P., Christie P.T. Modeling study of human renal chloride channel (hCLC-5) mutations suggests a structural-functional relationship. Kidney Int. 2003;63:1426–1432. doi: 10.1046/j.1523-1755.2003.00859.x. [DOI] [PubMed] [Google Scholar]
- 6.Blanchard A., Curis E., Guyon-Roger T. Observations of a large Dent disease cohort. Kidney Int. 2016;90:430–439. doi: 10.1016/j.kint.2016.04.022. [DOI] [PubMed] [Google Scholar]
- 7.Fervenza F.C. A patient with nephrotic-range proteinuria and focal global glomerulosclerosis. Clin J Am Soc Nephrol. 2013;8:1979–1987. doi: 10.2215/CJN.03400313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Claverie-Martin F., Ramos-Trujillo E., Garcia-Nieto V. Dent's disease: clinical features and molecular basis. Pediatr Nephrol. 2011;26:693–704. doi: 10.1007/s00467-010-1657-0. [DOI] [PubMed] [Google Scholar]
- 9.Devuyst O., Christie P.T., Courtoy P.J. Intra-renal and subcellular distribution of the human chloride channel, CLC-5, reveals a pathophysiological basis for Dent's disease. Human Mol Gen. 1999;8:247–257. doi: 10.1093/hmg/8.2.247. [DOI] [PubMed] [Google Scholar]
- 10.Kriz W. The pathogenesis of 'classic' focal segmental glomerulosclerosis-lessons from rat models. Nephrol Dial Transplant. 2003;18 Suppl 6:vi39–vi44. doi: 10.1093/ndt/gfg1064. [DOI] [PubMed] [Google Scholar]
- 11.Asanuma K., Mundel P. The role of podocytes in glomerular pathobiology. Clin Exp Nephrol. 2003;7:255–259. doi: 10.1007/s10157-003-0259-6. [DOI] [PubMed] [Google Scholar]
- 12.Pollak M.R. Familial FSGS. Adv Chronic Kidney Dis. 2014;21:422–425. doi: 10.1053/j.ackd.2014.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dickson L.E., Wagner M.C., Sandoval R.M., Molitoris B.A. The proximal tubule and albuminuria: really! J Am Soc Nephrol. 2014;25:443–453. doi: 10.1681/ASN.2013090950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grgic I., Campanholle G., Bijol V. Targeted proximal tubule injury triggers interstitial fibrosis and glomerulosclerosis. Kidney Int. 2012;82:172–183. doi: 10.1038/ki.2012.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arif E., Sharma P., Solanki A. Structural analysis of the Myo1c and Neph1 complex provides insight into the intracellular movement of Neph1. Mol Cell Biol. 2016;36:1639–1654. doi: 10.1128/MCB.00020-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ceresa B.P., Lotscher M., Schmid S.L. Receptor and membrane recycling can occur with unaltered efficiency despite dramatic Rab5(Q79L)-induced changes in endosome geometry. J Biolog Chem. 2001;276:9649–9654. doi: 10.1074/jbc.M010387200. [DOI] [PubMed] [Google Scholar]
- 17.Krendel M., Osterweil E.K., Mooseker M.S. Myosin 1E interacts with synaptojanin-1 and dynamin and is involved in endocytosis. FEBS Lett. 2007;581:644–650. doi: 10.1016/j.febslet.2007.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ding F., Li X., Li B. Calpain-mediated cleavage of calcineurin in puromycin aminonucleoside-induced podocyte injury. PloS One. 2016;11:e0155504. doi: 10.1371/journal.pone.0155504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.George B., Vollenbröker B., Saleem M.A. GSK3β inactivation in podocytes results in decreased phosphorylation of p70S6K accompanied by cytoskeletal rearrangements and inhibited motility. A J Physiol Renal Physiol. 2011;300:F1152–F1162. doi: 10.1152/ajprenal.00373.2010. [DOI] [PubMed] [Google Scholar]
- 20.Arif E., Rathore Y.S., Kumari B. Slit diaphragm protein Neph1 and its signaling: a novel therapeutic target for protection of podocytes against glomerular injury. J Biol Chem. 2014;289:9502–9518. doi: 10.1074/jbc.M113.505743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ceol M., Tiralongo E., Baelde H.J. Involvement of the tubular ClC-type exchanger ClC-5 in glomeruli of human proteinuric nephropathies. PloS One. 2012;7 doi: 10.1371/journal.pone.0045605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gianesello L., Priante G., Ceol M. Albumin uptake in human podocytes: a possible role for the cubilin-amnionless (CUBAM) complex. Sci Rep. 2017;7:13705. doi: 10.1038/s41598-017-13789-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang Y., Cai H., Cebotaru L. ClC-5: role in endocytosis in the proximal tubule. Am J Physiol Renal Physiol. 2005;289:F850–F862. doi: 10.1152/ajprenal.00011.2005. [DOI] [PubMed] [Google Scholar]
- 24.Piwon N., Gunther W., Schwake M. ClC-5 Cl–channel disruption impairs endocytosis in a mouse model for Dent's disease. Nature. 2000;408:369–373. doi: 10.1038/35042597. [DOI] [PubMed] [Google Scholar]
- 25.Sethi S.K., Otto E.A., Ma S. A boy with proteinuria and focal global glomerulosclerosis: question and answers. Pediatr Nephrol. 2015;30:1945–1949. doi: 10.1007/s00467-014-2959-4. [DOI] [PubMed] [Google Scholar]
- 26.Cramer M.T., Charlton J.R., Fogo A.B. Expanding the phenotype of proteinuria in Dent disease. A case series. Pediatr Nephrol. 2014;29:2051–2054. doi: 10.1007/s00467-014-2824-5. [DOI] [PubMed] [Google Scholar]
- 27.Ludwig M., Doroszewicz J., Seyberth H.W. Functional evaluation of Dent's disease-causing mutations: implications for ClC-5 channel trafficking and internalization. Human Gen. 2005;117:228–237. doi: 10.1007/s00439-005-1303-2. [DOI] [PubMed] [Google Scholar]
- 28.Laudrel S., Grand T., Burgos J. ClC5 mutations associated with Dent's disease: a major role of the dimer interface. Pflugers Arch. 2012;463:247–256. doi: 10.1007/s00424-011-1052-0. [DOI] [PubMed] [Google Scholar]
- 29.Wang X., Anglani F., Beara-Lasic L. Glomerular pathology in Dent disease and its association with kidney function. Clin J Am Soc Nephrol. 2016;11:2168–2176. doi: 10.2215/CJN.03710416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Inoue K., Ishibe S. Podocyte endocytosis in the regulation of the glomerular filtration barrier. Am J Physiol Renal Physiol. 2015;309:F398–F405. doi: 10.1152/ajprenal.00136.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gorvin C.M., Wilmer M.J., Piret S.E. Receptor-mediated endocytosis and endosomal acidification is impaired in proximal tubule epithelial cells of Dent disease patients. Proc Natl Acad Sci U S A. 2013;110:7014–7019. doi: 10.1073/pnas.1302063110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reiser J., Oh J., Shirato I. Podocyte migration during nephrotic syndrome requires a coordinated interplay between cathepsin L and alpha3 integrin. J Biol Chem. 2004;279:34827–34832. doi: 10.1074/jbc.M401973200. [DOI] [PubMed] [Google Scholar]
- 33.Sever S., Altintas M.M., Nankoe S.R. Proteolytic processing of dynamin by cytoplasmic cathepsin L is a mechanism for proteinuric kidney disease. J Clin Investig. 2007;117:2095–2104. doi: 10.1172/JCI32022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bullich G., Vargas I., Trujillano D. Contribution of the TTC21B gene to glomerular and cystic kidney diseases. Nephrol Dial Transplant. 2017;32:151–156. doi: 10.1093/ndt/gfv453. [DOI] [PubMed] [Google Scholar]
- 35.Cong E.H., Bizet A.A., Boyer O. A homozygous missense mutation in the ciliary gene TTC21B causes familial FSGS. J Am Soc Nephrol. 2014;25:2435–2443. doi: 10.1681/ASN.2013101126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Valina M.R., Larsen C.P., Kanosky S. A novel CLCN5 mutation in a boy with asymptomatic proteinuria and focal global glomerulosclerosis. Clin Nephrol. 2013;80:377–384. doi: 10.5414/CN107429. [DOI] [PubMed] [Google Scholar]